Exploring supercapacitors to improve their structure
No matter how intimidating their name, supercapacitors are part of our daily lives. Take buses for example: supercapacitors are charged during braking, and supply electricity to open the doors when the vehicle stops. Yet the molecular organization and functioning of these electricity storage devices had not previously been observed. For the first time, researchers from CNRS and the Université d'Orléans have explored the molecular rearrangements at play in commercially available supercapacitors while in operation. The technique devised by the scientists provides a new tool for optimizing and improving tomorrow's supercapacitors. The results are published on-line on Nature Materials' website on 17 February 2013.
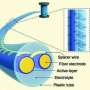
Supercapacitors are electricity storage devices that are quite different from batteries. Unlike batteries, supercapacitors are charged much faster (usually in seconds), and do not suffer rapid wear due to charging/discharging. On the other hand, at equivalent size and although they offer greater power, they cannot store as much electrical energy as batteries (carbon-based supercapacitors supply an energy density of around 5 Wh/kg compared to around 100 Wh/kg for lithium-ion batteries). Supercapacitors are used in the recovery of braking energy in numerous vehicles (cars, buses, trains, etc.) and to open the emergency exits of the Airbus A380.
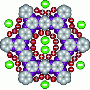
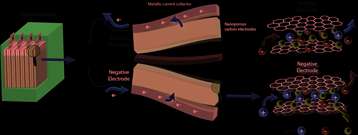
A supercapacitor stores electricity through the interaction between nanoporous carbon electrodes and ions, which carry positive and negative charges, and move about in a liquid known as an electrolyte. When charging, the anions (negatively charged ions) are replaced by cations (positively charged ions) in the negative electrode and vice versa. The greater this exchange and the higher the available carbon surface area, the greater the capacity of the supercapacitor.
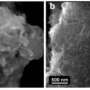
Using Nuclear Magnetic Resonance (NMR) spectroscopy, researchers delved deeper into this phenomenon and were able, for the first time, to quantify the proportion in which charge exchanges take place in two supercapacitors using commercially available carbons. By comparing two nanoporous carbon materials, the researchers were able to show that the supercapacitor containing the carbon with the most disordered structure had greater capacitance and improved high-voltage tolerance. This could be due to better electronic charge distribution upon contact with the electrolyte molecules.
More information: Deschamps, M. et al. Exploring electrolyte organization in supercapacitor electrodes with solid-state NMR, Nature Materials. Published on-line on the 17 February DOI: 10.1038/NMAT3567.
Journal information: Nature Materials
Provided by CNRS