Penicillin redux: Rearming proven warriors for the 21st century
Penicillin, one of the scientific marvels of the 20th century, is currently losing a lot of battles it once won against bacterial infections. But scientists at the University of South Carolina have just reported a new approach to restoring its combat effectiveness, even against so-called "superbugs."
Bacteria have been chipping away at the power of the penicillin family of drugs since their first wide-scale use as antibiotics in the 1940s. For example, the staph infection, brought about by the bacterium Staphylococcus aureus, was once readily treated with penicillin and its molecular cousins.
But that bug has changed. In the 1960s, a new strain arrived, termed MRSA for methicillin- (or sometimes multidrug-) resistant S. aureus. It has become a serious public health problem because the earliest deployed antibiotics are often useless against the new strain, and its prevalence has only increased since it was first observed. MRSA (pronounced mer-suh) is sometimes called a superbug because of the difficulty physicians have in treating infected patients.
The S. aureus microbe has evolved the MRSA strain by developing a variety of defenses against antibiotics to which they've been exposed. One of those defenses effectively neutralizes penicillin's greatest strength.
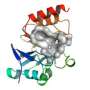
That strength is its molecular core, a cyclic four-membered amide ring termed a beta-lactam. It is a common structural element of the penicillins, their synthetic and semi-synthetic derivatives, and other related molecules that constitute the broad family of drugs called the beta-lactam antibiotics. Just a few examples (of dozens) include amoxicillin, ampicillin and cefazolin.
The beta-lactam structure in a molecule is something that many bacteria don't like at all. It greatly hinders their ability to reproduce by cell division, and so chemists have for years spent time making molecules that all contain the beta-lactam structural motif, but differ in the surrounding molecular "shrubbery." Physicians heavily use the many versions of beta-lactam antibiotics to fight bacterial infections, and many have been retired because they're no longer effective against the defenses bacteria have evolved in response.
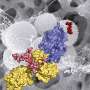
One of the most effective bacterial defenses is an enzyme called beta-lactamase, which chews up the beta-lactam structure. Some bacteria, such as MRSA, have developed the ability to biosynthesize and release beta-lactamase when needed. It's a devastating defense because it's so general, targeting the common structural motif in all of the many beta-lactam antibiotics.
But that also creates the opportunity for a general approach to solving the problem, which is what Carolina's Chuanbing Tang and colleagues just reported in the Journal of the American Chemical Society.
"Instead of developing new antibiotics, here we ask the question, 'can we recycle the old antibiotics?' " he said. "With traditional antibiotics like penicillin G, amoxicillin, ampicillin and so on, can we give them new life?"
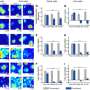
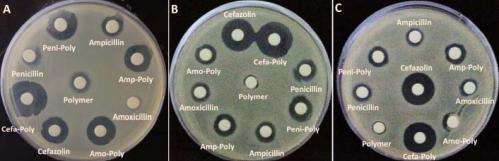
The approach pairs the drug with a protective polymer developed in Tang's chemistry laboratory. In lab tests, graduate student Jiuyang Zhang prepared a cobaltocenium metallopolymer that greatly slowed the destructiveness of beta-lactamase on a model beta-lactam molecule (nitrocefin).
The interdisciplinary team, which included Mitzi Nagarkatti and Alan Decho, from the university's School of Medicine and Arnold School of Public Health, respectively, also showed that the antimicrobial effectiveness of the four beta-lactams studied in detail was enhanced by the polymer. The enhancement was modest against two strains, but very pronounced with the hospital-associated strain of MRSA (HA-MRSA).
The metallopolymer by itself even demonstrated antimicrobial properties, lysing bacterial cells while leaving human red blood cells unaffected. By a variety of measures, the polymer was found to be nontoxic to human cells in laboratory tests.
The project is still far from clinical use, but Tang knows moving forward is imperative.
"In the United States every year, around 100,000 patients die of bacteria-induced infections," Tang said. "And the problem is increasing because bacteria are building resistance.
"It's a really, really big problem, not only for individual patients, but also for society."
More information: Paper: dx.doi.org/10.1021/ja5011338
Journal information: Journal of the American Chemical Society
Provided by University of South Carolina