Research team implants human innate immune cells in mice
(Phys.org) —Overcoming a major limitation to the study of the origins and progress of human disease, Yale researchers report that they have transplanted human innate immune cells into mouse models, which resulted in human immune responses. This groundbreaking study has reproduced human immune function at a level not seen previously, and could significantly improve the translation of knowledge gained from mouse studies into humans. The study is published online in Nature Biotechnology.
The innate immune system is the body's first line of defense against bacteria and viruses, and provides the adaptive immune system with the necessary information to create custom-made B and T cells that target specific bacterial or viral pathogens. Humanized mice are often used for studies of human immune responses, but until now the translation has been imperfect because existing mouse models do not permit efficient development of human innate immune cells.
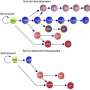
The Yale team was able to overcome this obstacle by combining human versions of four genes encoding cytokines—proteins that support immune cell development—when transplanting a human immune system into the mouse. These cytokines support the development and functions of many elements of the innate immune system.
Further, the researchers observed that transplanted disease-fighting human macrophage cells were able to infiltrate a human tumor graft in the mouse strains in a manner similar to what happens in human patients. The authors report that the mice demonstrated a human innate immune response that is essential for early response to foreign invaders.
"It was a lengthy effort to express all those human genes in the mouse, and then to transplant a human immune system," said first author Anthony Rongvaux, associate research scientist in the Department of Immunobiology at Yale School of Medicine. "But in the end, the result is remarkable. This new model will now allow us to address important questions that remain unanswered about how the human immune system fights infection and cancer."
"This humanized innate immune system may prove extremely valuable in studying human health and pathology, and may lead to development of new therapies for human disease," said senior author Richard Flavell, chair and Sterling Professor of Immunobiology at Yale School of Medicine, a member of Yale Cancer Center, and a Howard Hughes Medical Institute investigator.
More information:
"Development and function of human innate immune cells in a humanized mouse model," Anthony Rongvaux, et al. Nature Biotechnology (2014) DOI: 10.1038/nbt.2858
Received 21 October 2013 Accepted 24 February 2014 Published online 16 March 2014
Journal information: Nature Biotechnology
Provided by Yale University