Researchers turn a nickel-rich nanoparticle into a platinum-rich "nanoframe"
(Phys.org) —For hundreds of years, alchemists have tried to turn base metals into precious ones. Though they may never turn lead into gold, scientists have discovered a way to turn a nickel-rich nanoparticle into a platinum-rich "nanoframe" that could shape the development of fuel cells and other electrochemical technologies.
Researchers at the U.S. Department of Energy's Argonne National Laboratory and Lawrence Berkeley National Laboratory teamed up to convert platinum-nickel polyhedra into bare frames that had a much richer platinum content. Argonne physical chemist Vojislav Stamenkovic, and Lawrence Berkeley researcher and UC Berkeley professor Peidong Yang led the research team that has given scientists a new approach to catalysis.
"Polyhedra have been the usual nanostructures used for decades for catalysis research," Stamenkovic said. "Our research shows that there may be other options available."
Platinum is a highly active catalytic agent, making it desirable for researchers who are searching for new materials for fuel cells and metal-air batteries, among other technologies. Unfortunately, because of its rare and expensive nature, researchers have had to find ways to use it as efficiently as possible. In the polyhedron configuration, many of the prized platinum atoms were buried and unreachable in the bulk of the nanoparticle.
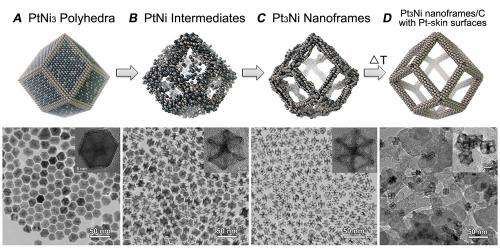
By eroding the inside of the nanoparticle using a chemical process, the researchers were able to create a nanoframe – a skeleton of the original polyhedron that retained the relatively platinum-rich edges. While the original polyhedron consisted of three nickel atoms for every platinum one, the nanoframes had, on average, the reverse proportions.
The choice to use nanoframes as opposed to polyhedra conferred on the researchers an additional major advantage. Instead of having to come into contact with the surface of the nanoparticle, catalyzed molecules could contact it from any direction at all – including what used to be the inside of the structure. This increased the surface area available for reactions to take place.
"With frames, we completely opened the structure and got rid of the buried nonfunctional bulk atoms. There are still a substantial number of active sites on the nanoframes that can be approached from any direction," Stamenkovic said.
After eroding the material, the Argonne and Berkeley scientists wanted to ensure its stability in the harsh, highly demanding electrochemical environment. To do so, they created a "second skin" of platinum over the nanoframe, increasing its durability.
According to Yang, the nanocatalyst frames offer a number of advantages. "In contrast to other synthesis procedures for hollow nanostructures that involve corrosion induced by harsh oxidizing agents or applied potential, our method proceeds spontaneously in air," he said. "The open structure of our platinum/nickel nanoframes addresses some of the major design criteria for advanced nanoscale electrocatalysts, including high surface-to-volume ratio, 3-D surface molecular accessibility and significantly reduced precious metal utilization."
"Our results describe a new class of materials based on the hollow nanoframe's open architecture and its well-defined surface compositional profile," Stamenkovic added. "The technique for making these hollow nanoframes can be readily applied to other multimetallic electrocatalysts or gas phase catalysts. We are quite optimistic about its commercial viability."
A paper based on the research entitled "Highly Crystalline Multimetallic Nanoframes with Three-Dimensional Electrocatalytic Surfaces" appears in the February 27 edition of Science Express and will be published soon in Science.
More information: "Highly Crystalline Multimetallic Nanoframes with Three-Dimensional Electrocatalytic Surfaces." Chen Chen, Yijin Kang, Ziyang Huo, Zhongwei Zhu, Wenyu Huang, Huolin L. Xin, Joshua D. Snyder, Dongguo Li, Jeffrey A. Herron, Manos Mavrikakis, Miaofang Chi, Karren L. More, Yadong Li, Nenad M. Markovic, Gabor A. Somorjai, Peidong Yang, and Vojislav R. Stamenkovic. Science 1249061Published online 27 February 2014 [DOI: 10.1126/science.1249061]
Journal information: Science Express , Science
Provided by Argonne National Laboratory