Cobalt catalysts allow researchers to duplicate the complicated steps of photosynthesis

(Phys.org) —Humans have for ages taken cues from nature to build their own devices, but duplicating the steps in the complicated electronic dance of photosynthesis remains one of the biggest challenges and opportunities for chemists.
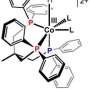
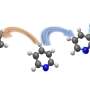
Currently, the most efficient methods we have for making fuel – principally, hydrogen – from sunlight and water involve rare and expensive metal catalysts, such as platinum. In a new study, researchers at the U.S. Department of Energy's Argonne National Laboratory have found a new, more efficient way to link a less expensive synthetic cobalt-containing catalyst to an organic light-sensitive molecule, called a chromophore.
Although cobalt is significantly less efficient than platinum when it comes to light-induced hydrogen generation, the drastic price difference between the two metals makes cobalt the obvious choice as the foundation for a synthetic catalyst, said Argonne chemist Karen Mulfort.
"Cobalt doesn't have to be as efficient as platinum because it is just so much cheaper," she said.
The Argonne study wasn't the first to look at cobalt as a potential catalytic material; however, the paper did identify a new mechanism by which to link the chromophore with the catalyst. Previous experiments with cobalt attempted to connect the chromophore directly with the cobalt atom within the larger compound, but this eventually caused the hydrogen generation process to break down.
Instead, the Argonne researchers connected the chromophore to part of a larger organic ring that surrounded the cobalt atom, which allowed the reaction to continue significantly longer.
"If we were to directly link the chromophore and the cobalt atom, many of the stimulated electrons quickly fall out of the excited state back into the ground state before the energy transfer can occur," Mulfort said. "By coupling the two materials in the way we've described, we can have much more confidence that the electrons are going to behave the way we want them to."
One additional advantage of working with a cobalt-based catalyst, in addition to its relatively low price and abundance, is the fact that scientists understand the atomic-level mechanisms at play.
"There's a lot of different ways in which we already know we can modify cobalt-based catalysts, which is important because we need to make our devices more robust," Mulfort said.
Future studies in this arena could involve nickel- and iron-based catalysts – metals which are even more naturally abundant than cobalt, although they are not quite as effective natural catalysts. "We want to extrapolate from what we've gained by looking at this kind of linkage in respect to other catalysts," Mulfort said.
Mulfort and her Argonne colleagues used the high-intensity X-rays provided by the laboratory's Advanced Photon Source as well as precise spectroscopic techniques available at Argonne's Center for Nanoscale Materials.
A paper based on the study appeared in the journal Physical Chemistry Chemical Physics. The research was supported by DOE's Office of Science.
Journal information: Physical Chemistry Chemical Physics
Provided by Argonne National Laboratory