How zinc starves lethal bacteria to stop infection
(Phys.org) —Australian researchers have found that zinc can 'starve' one of the world's most deadly bacteria by preventing its uptake of an essential metal.
The finding, by infectious disease researchers at the University of Adelaide and The University of Queensland, opens the way for further work to design antibacterial agents in the fight against Streptococcus pneumoniae.
Streptococcus pneumoniae is responsible for more than one million deaths a year, killing children, the elderly and other vulnerable people by causing pneumonia, meningitis, and other serious infectious diseases.
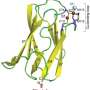
Published today in the journal Nature Chemical Biology, the researchers describe how zinc "jams shut" a protein transporter in the bacteria so that it cannot take up manganese, an essential metal that Streptococcus pneumoniae needs to be able to invade and cause disease in humans.
"It's long been known that zinc plays an important role in the body's ability to protect against bacterial infection, but this is the first time anyone has been able to show how zinc actually blocks an essential pathway causing the bacteria to starve," says project leader Dr Christopher McDevitt, Research Fellow in the University of Adelaide's Research Centre for Infectious Diseases.
"This work spans fields from chemistry and biochemistry to microbiology and immunology to see, at an atomic level of detail, how this transport protein is responsible for keeping the bacteria alive by scavenging one essential metal (manganese), but at the same time also makes the bacteria vulnerable to being killed by another metal (zinc)," says Professor Bostjan Kobe, Professor of Structural Biology at The University of Queensland.
The study reveals that the bacterial transporter (PsaBCA) uses a 'spring-hammer' mechanism to bind the metals. The difference in size between the two metals, manganese and zinc, causes the transporter to bind them in different ways. The smaller size of zinc means that when it binds to the transporter, the mechanism closes too tightly around the zinc, causing an essential spring in the protein to unwind too far, jamming it shut and blocking the transporter from being able to take up manganese.
"Without manganese, these bacteria can easily be cleared by the immune system," says Dr McDevitt. "For the first time, we understand how these types of transporters function. With this new information we can start to design the next generation of antibacterial agents to target and block these essential transporters."
More information: www.nature.com/nchembio/journa … l/nchembio.1382.html
Journal information: Nature Chemical Biology
Provided by University of Adelaide