Reversible wound closure: Dissolvable dendritic thioester hydrogel for sealing wounds
In first-aid situations, wounds must be quickly and effectively closed to stop blood loss and prevent infection. For treatment on arrival in a hospital, the temporary seal must be reopened, which often causes additional damage to the injured tissue. In the journal Angewandte Chemie, American scientists have now introduced a novel gel for sealing wounds. The gel can later be dissolved and gently removed.
Injuries sustained in remote areas, far from civilization, or in military action can often not be treated in a clinic until hours later. In such scenarios, a temporary wound closure system is desirable. Such a system should: 1) stop the bleeding for several hours, 2) adhere to the tissue, 3) be easy to apply, and 4) be easily removable in a controlled manner to make the wound accessible during surgical treatment. No single wound-closure systems currently available meet all of these requirements. Removal of blood-clotting agents or dressings requires tearing or surgical excision, both of which can increase the size of the wound and make it worse.
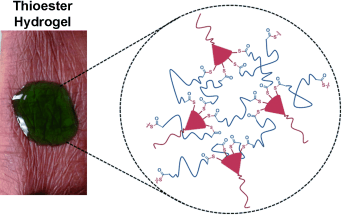
Scientists working at Boston University and the Beth Israel Deaconess Medical Center in Boston have now developed a wound-closure system based on a synthetic biocompatible gel that meets the requirements listed above. The gel is cross-linked through branched thioesters. The team, led by Mark W. Grinstaff, uses a chemical reaction known as thiol–thioester exchange in order to dissolve these gels for removal. In this reaction, a thioester bond reacts with a thiolate anion to produce new thioester and thiolate products. The advantage of this reaction is that it takes place in an aqueous environment under physiological conditions. This type of reaction also occurs in natural biological processes. When the thioester gel is treated with cysteine methyl ester, the thioester bridges are rapidly split and the gel dissolves.
Wounds may be treated by simply mixing and applying two starting materials. The gel forms within seconds, adheres to the skin even when stress is applied and remains intact for several days. The gel absorbs any liquid exiting the wound. Treatment with cysteine methyl ester causes the wound closure to reopen within 30 minutes. To simulate injury to a vein, the researchers filled a section of bovine jugular vein with buffer solution and punctured it. Once the gel was applied, the damaged vein was completely sealed; after dissolution of the gel, the buffer solution flowed out again.
More information: Mark W. Grinstaff, A Dendritic Thioester Hydrogel Based on Thiol–Thioester Exchange as a Dissolvable Sealant System for Wound Closure, Angewandte Chemie International Edition, dx.doi.org/10.1002/anie.201308007
Journal information: Angewandte Chemie , Angewandte Chemie International Edition
Provided by Wiley
















