Mechanism of the sodium-potassium pump revealed
Researchers from Aarhus University have collaborated with a Japanese group of researchers to establish the structure of a crucial enzyme—the so-called sodium-potassium pump—which forms part of every cell in the human body. The result, which was recently published in Nature, may pave the way for a better understanding of neurological diseases.
It's not visible to the naked eye and you can't feel it, but up to 40 per cent of your body's energy goes into supplying the microscopic sodium-potassium pump with the energy it needs. The pump is constantly doing its job in every cell of all animals and humans. It works much like a small battery which, among other things, maintains the sodium balance which is crucial to keep muscles and nerves working.
The sodium-potassium pump transports sodium out and potassium into the cell in a fixed cycle. During this process the structure of the pump changes. It is well-established that the pump has a sodium and a potassium form. But the structural differences between the two forms have remained a mystery, and researchers have been unable to explain how the pump distinguishes sodium from potassium.
Structure solves the mystery
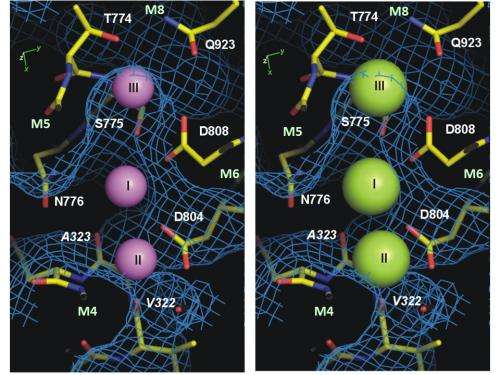
Thanks to the international collaboration between Professor Chikashi Toyoshima's group at the University of Tokyo and researchers from Aarhus University, the structure of the sodium-bound form of the protein has now been described. For the first time ever, the sodium ions can be studied at a resolution so high - 0.28 nanometres - that researchers can actually see the sodium ions and observe where they bind in the structure of the pump. In 2000, Professor Chikashi Toyoshima's group described the structure of a calcium-pump for the first time, and in 2007 and 2009 research groups from Aarhus University and Toyoshima's group described the potassium-bound form of the sodium-potassium pump.
"The new protein structure shows how the smaller sodium ions are bound and subsequently transported out of the cell, whereas the access of the slightly larger potassium ions is blocked. We now understand how the pump distinguishes between sodium and potassium at the molecular level. This is a great leap forward for research into ion pumps and may help us understand and treat serious neurological conditions associated with mutations of the sodium-potassium pump, including a form of Parkinsonism and alternating hemiplegia of childhood in which sodium binding is defective," explains Bente Vilsen, a professor at Aarhus University who spearheaded the project's activities in Aarhus with Associate Professor Flemming Cornelius.
Impressed Nobel Prize winner
The vital pump was discovered in 1957 by Professor Jens Christian Skou of Aarhus University, who received the Nobel Prize for his discovery in 1997. The new result is the culmination of five or six decades of research aimed at the mechanism behind this vital motor of the cells.
"Years ago, when the first electron microscopic images were taken in which the enzyme was but a millimetre-sized dot at 250,000 magnifications, I thought, how on earth will we ever be able to establish the structure of the enzyme. The pump transports potassium into and sodium out of the cells, so it must be capable of distinguishing between the two ions. But until now, it has been a mystery how this was possible," says retired Professor Jens Christian Skou, who - even at 94 years of age - keeps up to date with new developments in the field of research which he initiated more than 50 years ago.
"Now, the researchers have described the structure that allows the enzyme to identify sodium and this may pave the way for a more detailed understanding of how the pump works. It is an impressive achievement and something I haven't even dared dream of," concludes Jens Christian Skou.
More information: www.nature.com/nature/journal/ … ull/nature12578.html
Journal information: Nature
Provided by Aarhus University















