Mix of graphene nanoribbons, polymer has potential for cars, soda, beer
A discovery at Rice University aims to make vehicles that run on compressed natural gas more practical. It might also prolong the shelf life of bottled beer and soda.
The Rice lab of chemist James Tour has enhanced a polymer material to make it far more impermeable to pressurized gas and far lighter than the metal in tanks now used to contain the gas.
The combination could be a boon for an auto industry under pressure to market consumer cars that use cheaper natural gas. It could also find a market in food and beverage packaging.
Tour and his colleagues at Rice and in Hungary, Slovenia and India reported their results this week in the online edition of the American Chemistry Society journal ACS Nano.
By adding modified, single-atom-thick graphene nanoribbons (GNRs) to thermoplastic polyurethane (TPU), the Rice lab made it 1,000 times harder for gas molecules to escape, Tour said. That's due to the ribbons' even dispersion through the material. Because gas molecules cannot penetrate GNRs, they are faced with a "tortuous path" to freedom, he said.
The researchers acknowledged that a solid, two-dimensional sheet of graphene might be the perfect barrier to gas, but the production of graphene in such bulk quantities is not yet practical, Tour said.
But graphene nanoribbons are already there. Tour's breakthrough "unzipping" technique for turning multiwalled carbon nanotubes into GNRs, first revealed in Nature in 2009, has been licensed for industrial production. "These are being produced in bulk, which should also make containers cheaper," he said.
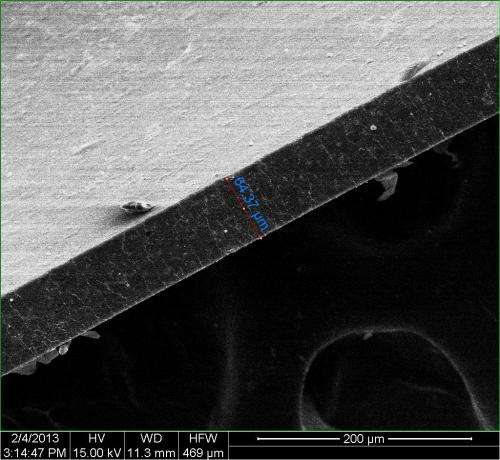
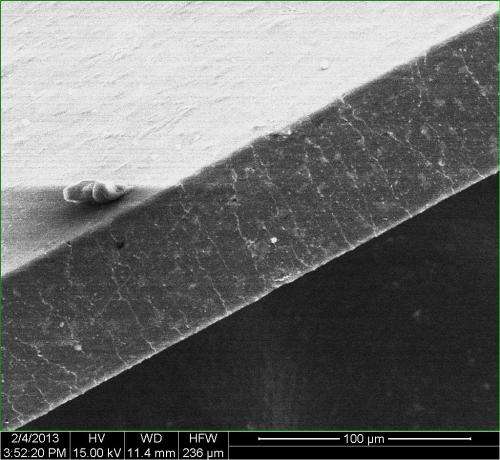
The researchers led by Rice graduate student Changsheng Xiang produced thin films of the composite material by solution casting GNRs treated with hexadecane and TPU, a block copolymer of polyurethane that combines hard and soft materials. The tiny amount of treated GNRs accounted for no more than 0.5 percent of the composite's weight. But the overlapping 200- to 300-nanometer-wide ribbons dispersed so well that they were nearly as effective as large-sheet graphene in containing gas molecules. The GNRs' geometry makes them far better than graphene sheets for processing into composites, Tour said.
They tested GNR/TPU films by putting pressurized nitrogen on one side and a vacuum on the other side. For films with no GNRs, the pressure dropped to zero in about 100 seconds as nitrogen escaped into the vacuum chamber. With GNRs at 0.5 percent, the pressure didn't budge over 1,000 seconds, and it dropped only slightly over more than 18 hours.
Stress and strain tests also found that the 0.5 percent ratio was optimal for enhancing the polymer's strength.
"The idea is to increase the toughness of the tank and make it impermeable to gas," Tour said. "This becomes increasingly important as automakers think about powering cars with natural gas. Metal tanks that can handle natural gas under pressure are often much heavier than the automakers would like."

He said the material could help to solve long-standing problems in food packaging, too.
"Remember when you were a kid, you'd get a balloon and it would be wilted the next day? That's because gas molecules go through rubber or plastic," Tour said. "It took years for scientists to figure out how to make a plastic bottle for soda. Once, you couldn't get a carbonated drink in anything but a glass bottle, until they figured out how to modify plastic to contain the carbon dioxide bubbles. And even now, bottled soda goes flat after a period of months.
"Beer has a bigger problem and, in some ways, it's the reverse problem," he said. "Oxygen molecules get in through plastic and make the beer go bad." Bottles that are effectively impermeable could lead to brew that stays fresh on the shelf for far longer, Tour said.
More information: pubs.acs.org/doi/abs/10.1021/nn404843n
Journal information: ACS Nano
Provided by Rice University