Finding could potentially make iPS cells safer for use in humans
Induced pluripotent stem cells, or iPS cells, are a hot commodity right now in biology.
The cells, which are created when non-stem cells are reprogrammed to resemble embryonic stem cells, have many potential uses in therapy and drug development. They're usually created by using a virus to add just four genes (selected because they are highly expressed in embryonic stem cells) to the cell to be reprogrammed.
However, a molecular understanding of the transformation process is largely lacking, and the expression of one of the genes, called c-Myc, is frequently elevated in human cancers. This has given researchers and clinicians pause when considering the use of iPS cells in humans.
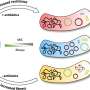
Now researchers in the laboratory of Helen Blau, PhD, the Donald E. and Delia B. Baxter Professor, have found a replacement for c-Myc. They did so by fusing mouse embryonic stem cells with a human skin cell, or fibroblast, to create a bi-species product, called a heterokaryon, with a 3:1 nuclear ratio, skewed toward the stem cells. This ratio shifts the balance of cellular proteins to favor pluripotency and turns out be an excellent way to study the earliest steps of reprogramming. That's because factors in the developmentally flexible stem cell nucleus reprogram the more-staid skin cell nucleus quickly and efficiently, giving researchers a ring-side seat to the intricate transformation process.
The vast majority of heterokaryons reprogram. This is in stark contrast with the only about one in every 1,000 would-be iPS cells that ever complete their transformation to pluripotency: a pretty uninformative show if you pick the wrong cell to follow.
"Studying these heterokaryons gives us a molecular snapshot of pluripotency that would otherwise have been missed and allows us to capture reprogramming in action," Blau said. "For the first time, we're able to identify critically important transient regulators that would be totally missed by current methods of study."
Blau is the senior author of the research, which was published Sept. 1 in Nature Cell Biology. Postdoctoral scholar Jennifer Brady, PhD, is the lead author.
As Blau predicted, the study of the heterokaryons paid off. The researchers found that a signaling molecule called IL-6 is turned on and highly expressed in the human fibroblast nucleus during the first few hours of reprogramming in the fused cells. This gave them an important clue, and they were then able to show that during the creation of iPS cells, temporary exposure to IL-6 can replace c-Myc.
The iPS cells created without c-Myc should be safer to use in human therapies. But this is just the tip of the iceberg. Much more can be learned from the heterokaryon model, Blau said.
"This method provides insights into the logic and timing of the reprogramming process that would not be possible by any other means," she said. "Really understanding this process is vital to getting safer and more efficient reprogramming to make iPS cells."
More information: Early role for IL-6 signalling during generation of induced pluripotent stem cells revealed by heterokaryon RNA-Seq, DOI: 10.1038/ncb2835
Journal information: Nature Cell Biology
Provided by Stanford University Medical Center