September 6, 2013 report
Researchers develop first iron-based catalyst that can reduce dinitrogen to ammonia in a solution
(Phys.org) —A trio of researchers at California Institute of Technology has developed an iron-based catalyst that allows for reducing dinitrogen (a normal nitrogen molecule with two atoms) to ammonia in a solution. In their paper published in the journal Nature, John Anderson, Jonathan Rittle and Jonas Peters describe the technique they developed that allows for converting nitrogen to ammonia using iron as a basis for a catalyst.
Chemists and biologists alike have, for several decades, been stymied in their attempts to understand exactly how it is that some plants (and bacteria) are able to extract nitrogen from the air and convert it to ammonia—a process called nitrogenases. Scientists know that enzymes are at the heart of the process and that all of them contain iron and other metals, but they've not been able to settle on which is responsible for the actual conversion process. In this new effort, the research team has built a strong argument for iron, by creating a catalyst based on it that is able to convert dinitrogen to ammonia. Their success marks the first time such a catalyst has been found that is able to do so.
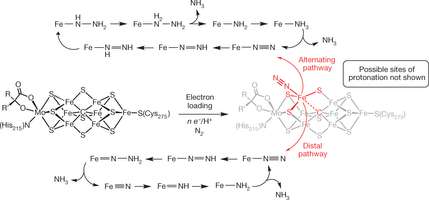
The catalyst they developed has a single iron atom that has been bound to a ligand that serves to prime the dinitrogen molecule. Electrons do the actual work of reducing the dinitrogen to ammonia—a quick process that uses only half of the electrons and protons in the mix.
The trios' results don't solve the mystery of nitrogenases, but they do perhaps offer a path to discovery. Prior methods have been developed that convert nitrogen to ammonia, but most rely on molybdenum along with a lot of heat and pressure. Commercial ammonia, for example, is mass produced using a method devised by Carl Bosch and Fritz Haber. In contrast, the process developed by the team at CIT runs at normal room temperature. They readily acknowledge that their method isn't going to supplant the Haber–Bosch process for making ammonia however—it's weak, not suitable at all for mass production. Each catalyst molecule was only able to convert seven nitrogen molecules before running out of steam.
More information: Catalytic conversion of nitrogen to ammonia by an iron model complex, Nature 501, 84–87 (05 September 2013) DOI: 10.1038/nature12435
Abstract
The reduction of nitrogen (N2) to ammonia (NH3) is a requisite transformation for life1. Although it is widely appreciated that the iron-rich cofactors of nitrogenase enzymes facilitate this transformation, how they do so remains poorly understood. A central element of debate has been the exact site or sites of N2 coordination and reduction. In synthetic inorganic chemistry, an early emphasis was placed on molybdenum8 because it was thought to be an essential element of nitrogenases3 and because it had been established that well-defined molybdenum model complexes could mediate the stoichiometric conversion of N2 to NH3. This chemical transformation can be performed in a catalytic fashion by two well-defined molecular systems that feature molybdenum centres. However, it is now thought that iron is the only transition metal essential to all nitrogenases, and recent biochemical and spectroscopic data have implicated iron instead of molybdenum as the site of N2 binding in the FeMo-cofacto. Here we describe a tris(phosphine)borane-supported iron complex that catalyses the reduction of N2 to NH3 under mild conditions, and in which more than 40 per cent of the proton and reducing equivalents are delivered to N2. Our results indicate that a single iron site may be capable of stabilizing the various NxHy intermediates generated during catalytic NH3 formation. Geometric tunability at iron imparted by a flexible iron–boron interaction in our model system seems to be important for efficient catalysis. We propose that the interstitial carbon atom recently assigned in the nitrogenase cofactor may have a similar role16, 17, perhaps by enabling a single iron site to mediate the enzymatic catalysis through a flexible iron–carbon interaction.
Journal information: Nature
© 2013 Phys.org