Computer simulations indicate calcium carbonate has a dense liquid phase (w/ Video)
Computer simulations conducted at the U.S. Department of Energy's Lawrence Berkeley National Laboratory (Berkeley Lab) could help scientists make sense of a recently observed and puzzling wrinkle in one of nature's most important chemical processes. It turns out that calcium carbonate—the ubiquitous compound that is a major component of seashells, limestone, concrete, antacids and myriad other naturally and industrially produced substances—may momentarily exist in liquid form as it crystallizes from solution.
Calcium carbonate is a huge player in the planet's carbon cycle, so any new insight into how it behaves is potentially big news. The prediction of a dense liquid phase during the conversion of calcium carbonate to a solid could help scientists understand the response of marine organisms to changes in seawater chemistry due to rising atmospheric CO2 levels. It could also help them predict the extent to which geological formations can act as carbon storage reservoirs, among other examples.
The research is published in the August 23 issue of the journal Science. It was performed in support of the Center for Nanoscale Control of Geologic CO2, an Energy Frontier Research Center established at Berkeley Lab by the U.S. Department of Energy.
The research may also reconcile some confounding experimental observations. For more than a century, scientists believed that crystals nucleate from solution by overcoming an energy barrier. But recent studies of calcium carbonate revealed the presence of nanoscopic clusters which, under certain conditions, appear to circumvent the barrier by following an alternative aggregation-based crystallization pathway.
"Because nucleation is ubiquitous in both natural and synthetic systems, those findings have forced diverse scientific communities to reevaluate their longstanding view of this process," says the study's co-corresponding author Jim De Yoreo, formerly of Berkeley Lab and now a scientist at Pacific Northwest National Laboratory.
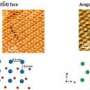
The Berkeley Lab-led team used molecular dynamics simulations to study the onset of calcium carbonate formation. The simulations predict that in sufficiently supersaturated calcium carbonate solutions, nanoscale dense liquid droplets can spontaneously form. These droplets then coalesce to form an amorphous solid prior to crystallization.
The findings support the aggregation-based mechanism of calcium carbonate formation. They also indicate that the presence of the nanoscale phase is consistent with a process called liquid-liquid separation, which is well known in alloys and polymers, but unexpected for salt solutions.
"Our simulations suggest the existence of a dense liquid form of calcium carbonate," says co-corresponding author Adam Wallace. He conducted the research while a post-doctoral researcher in Berkeley Lab's Earth Sciences Division, and is now an assistant professor in the Department of Geological Sciences at the University of Delaware.
"This is important because it is an as-yet unappreciated component of the carbon cycle," adds Wallace. "It also provides a means of explaining the unusual presence of nanoscale clusters in solution within the context of established physical mechanisms."
More information: The research, "Microscopic Evidence for Liquid-Liquid Separation in Supersaturated CaCO3 Solutions" is published in the August 23, 2013 issue of the journal Science.
Journal information: Science
Provided by Lawrence Berkeley National Laboratory