Breaking the code of royal purple

Royal purple, the color of robes swathing the emperors of Rome, ancient kings and high priests, and prized for its richness of hue and a brightness that wouldn't fade, has long carried its own molecular mystery.
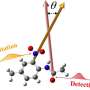
For years, chemists tried but failed to crack the code for the dye used to create Tyrian purple, named for the ancient city of Tyre where it was found. Solving its molecular structure held out the promise of important information for the textile industry and could lead to use of the indigo dye for medical fluorescent probes.
The original source of the highly valued dye had long been known—predatory sea snails—but the crystal structure of the indigo pigment, monobromoindigo, or MBI, remained a puzzle.
Intrigued, Baruch College chemistry student Olga Lavinda teamed with Baruch chemistry professor Keith Ramig, and together they were able to crystallize MBI for the first time and solve its structure, publishing their findings last year in the prestigious journal of X-ray crystal structures, Acta Crystallographica C.
"I was amazed that this was possible and it inspired me a lot," said Lavinda, who earned a bachelor's degree from Baruch in 2011 and is currently a Ph.D. fellow at New York University.
But her remarkable research on MBI would likely never have been possible if not for the work of CUNY Nobel laureate Jerome Karle, who died in June at age 94. Karle shared the 1985 Nobel Prize in Chemistry for creating the method for determining the X-ray crystal structures of molecules.
Karle's discovery revolutionized research into new drugs, paved the way for advances in medicine and other fields, and inspired countless young scientists including Lavinda.
"It's an immeasurable contribution," said Lavinda of Karle's work. "Virtually, all drug development is heavily reliant on X-ray crystallography. When you think of how many lives have been saved and how much progress we have made in just a small amount of time, it's hard to even quantify."
Hunter College chemistry professor Lou Massa, a colleague and close friend of Karle, said the Nobel Prize crystallized Karle's fame as one of the greatest scientists of his generation.
"Solving X-ray crystal structures … was for decades thought to be mathematically impossible," Massa said. "There is now almost no field of science and technology wherein crystal structures are not important."
Karle was born in Brooklyn, New York, in 1918, and attended Abraham Lincoln High School. At 15, he graduated and enrolled in City College. His student commute was grueling, he recalled in his autobiography, traveling three hours a day on the subway from Coney Island to City College and back.
After earning his undergraduate degree from City College in 1937, Karle received a master's degree in biology from Harvard University in 1938 and later a doctorate in chemistry from the University of Michigan, where he met his future wife and frequent laboratory partner, Isabella.
Near the end of World War II Karle worked at the University of Chicago on the Manhattan Project to develop the atomic bomb, focusing on the extraction and purification of plutonium.
In 1946, Karle and his wife moved to Washington, D.C., to work for the Naval Research Laboratory where he remained for more than 60 years, becoming the U.S. Navy's highest-ranking scientist. The following year, CCNY classmate and mathematician Herbert A. Hauptman joined the laboratory and they began working with X-rays and crystal structures.

X-ray crystallography had been discovered in 1912 by physicist Max von Laue as an important technique for displaying the atomic structure of a crystallized molecule by analyzing X-rays. But it was impossible to calculate exact structure because the difference in positions of scattered X-rays was unknown.
Karle and Hauptman's breakthrough was to devise a mathematical formula in X-ray crystallography for solving those unknown atomic positions. Their discovery, known as the "direct method," is now used by scientists worldwide to discern the structures and shapes of complex molecules.
"One of the fundamental ideas in chemistry is atomic structure," Massa said. "In medicine, when a drug is discovered the first question that is asked is: what is its structure? So, Karle's legacy is in providing the mathematics to solve that structure. And that is an amazing discovery."
When Karle and Hauptman published their discovery in the early 1950s, it was met with some opposition by scientists who weren't sure it worked. In his autobiography on the Nobel website, Karle recalled with frustration "a large number of fellow scientists did not believe a single word we said."
With increased use of computers in the 1970s, the "direct method" was confirmed and gained wide acceptance. The process of discerning the structure of three-dimensional molecules, which had sometimes taken months or years, could now be completed in a matter of days.
According to the Naval Research Laboratory, Karle's work enabled the characterization of potent toxins, antitoxins, heart drugs, antibiotics, antiaddictive substances, anticarcinogens, anti-malarials, and explosives and propellants.
Today, scientists, like Ramig, stand in awe of Karle for his brilliance and perseverance.
"When we say, here is the structure of a molecule, it has to be based on a certain method of analysis and X-ray crystallography is the only direct method where you can almost see the atoms," Ramig explained.
"So, Karle wrote the mathematics for solving the crystal structures. In those days, back in the 50s, they did calculations by hand, and the mathematics is incredibly complex. But Karle was able to do it."
In more recent research, Karle, along with Massa and CUNY Ph.D. graduate student Lulu Huang, created a new field known as quantum crystallography that combined X-ray diffraction data for crystals with quantum mechanics, according to the Naval laboratory.
Aside from his groundbreaking research, Karle was also known for encouraging young people to devote their lives to science. That interest in mentoring future scientists grew after he received the Nobel Prize, Massa said.
"He loved talking about science to young people," Massa said. "Youngsters wrote to him from faraway places. He not only wrote back, he wrote to their parents, too, with his best advice for supporting curiosity and freedom of thought in their children."
Karle would have appreciated Lavinda's intelligence and skill in solving the problem of the purple-dye molecule.
Massa said he met Lavinda when she came to Hunter to study physical chemistry. While they were having coffee one day, Massa told her about the difficulty in crystallizing the MBI molecule.
During their conversation, Massa showed Lavinda a research paper related to MBI and explained how several professional synthetic chemists had failed to crystallize the molecule. Lavinda, a Ukrainian native, stared at the line drawing of the MBI molecule in the paper.
She then said matter of factly: "Oh, that does not look too hard. Would you mind if I tried to crystallize the molecule for you?"
Within a few weeks, she and Ramig had made the crystals, using a method known as high-temperature recrystallization. Dr. David Szalda, a Baruch chemistry professor, used the crystals to solve the structure.
For Lavinda, the research with the indigo-like molecules formed an important part of her career because it allowed her to learn about crystallography, as well as origins of color, both fascinating branches of chemistry.
But the research also made her realize the extraordinary nature of Karle's work, she said.
"It blew my mind that somebody could mathematically define or describe the positions of atoms in space," Lavinda said.
"He had to have this whole imagination to see atoms and understand that every atom has its own charges and how they are influencing each other and how to calculate the prediction of where those atoms have to be. That to me is absolutely mind-boggling."
Provided by The City University of New York