Cobalt replacements make solar cells more sustainable
Researchers at the University of Basel have successfully replaced the rare element iodine in copper-based dye-sensitized solar cells by the more abundant element cobalt, taking a step forward in the development of environmentally friendly energy production. The journal Chemical Communications has published the results of these so-called Cu-Co cells.
Dye-sensitized solar cells (DSCs) transform light to electricity. They consist of a semiconductor on which a dye is anchored. This colored complex absorbs light and through an electron transfer process produces electrical current. Electrolytes act as electron transport agents inside the DSCs.
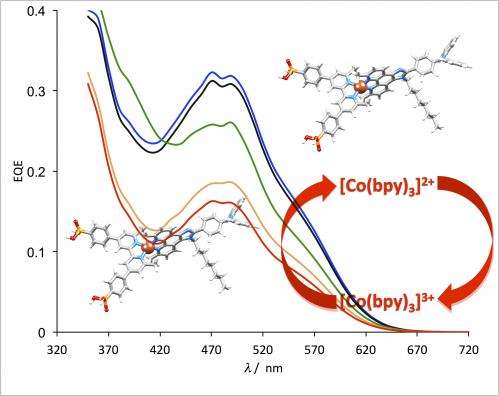
Usually, iodine and iodide serve as an electrolyte. Chemists at the University of Basel have now been able to successfully replace the usual iodine-based electron transport system in copper-based DSCs by a cobalt compound. Tests showed no loss in performance.
The replacement of iodine significantly increases the sustainability of solar cells: "Iodine is a rare element, only present at a level of 450 parts per billion in the Earth, whereas cobalt is 50 times more abundant", explains the Project Officer Dr. Biljana Bozic-Weber. Furthermore, this replacement also removes one of the long-term degradation processes in which copper compounds react with the electrolyte to form copper iodide and thus improves the long-term stability of DSCs.
The research group around the Basel chemistry professors Ed Constable and Catherine Housecroft is currently working on optimizing the performance of DSCs based on copper complexes. They had previously shown in 2012 that the very rare element ruthenium in solar cells could be replaced by copper derivatives.
This is the first report of DSCs, which combine copper-based dyes and cobalt electrolytes and thus represents a critical step towards the development of stable iodide-free copper solar cells. However, many aspects relating to the efficiency need to be addressed before commercialization can begin in anything other than niche markets.
Molecular systems engineering
"In changing any one component of these solar cells, it is necessary to optimize all other parts as a consequence", says Ed Constable. This is part of a new approach termed "Molecular Systems Engineering" in which all molecular and material components of a system can be integrated and optimized to approach new levels of sophistication in nanoscale machinery. In this publication, the engineering of the electrolyte, the dye and the semiconductor are all described.
This systems chemistry approach is particularly appropriate for the engineering of inorganic-biological hybrids and is the basis of ongoing collaborations with the ETH Department of Biosystems Engineering in Basel (D-BSSE) and EMPA. A joint proposal by the University of Basel and D-BSSE for a new National Centre of Competence in Research in this area is currently in the final stages of appraisal.
More information: Bozic-Weber, B. et al. Copper(I) dye-sensitized solar cells with [Co(bpy)3]2 /3 electrolyte, Chem. Commun., 2013,49, 7222-7224. DOI: 10.1039/C3CC44595J
Journal information: Chemical Communications
Provided by University of Basel