Bio-assisted nanophotocatalyst for hydrogen production
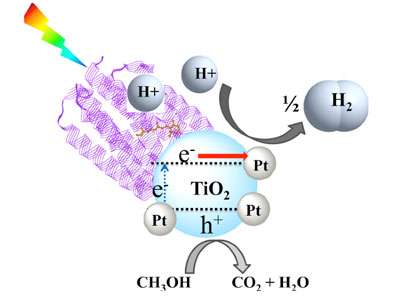
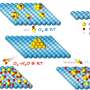
A protein found in the membranes of ancient microorganisms that live in desert salt flats could offer a new way of using sunlight to generate environmentally friendly hydrogen fuel. Researchers in the Nanobio Interfaces and Nanophotonics groups at Argonne National Laboratory combined the light-harvesting proton pump bacteriorhodopsin (bR) on a Pt/TiO2 nanocatalyst for visible light-driven hydrogen generation. The platinum nanocatalyst matrix is comprised of bR and 4 nm Pt(0) nanoparticles photodeposited on the surface of 25-nm TiO2 nanoparticles. Photoelectrochemical and transient absorption studies indicate efficient charge transfer between bR protein molecules and titania nanoparticles.
Scientists have been aware of the potential of TiO2 nanoparticles for light-based reactions since the early 1970s, when Japanese researchers discovered that a TiO2 electrode exposed to bright ultraviolet light could split water molecules in a phenomenon that came to be known as the Honda-Fujishima effect. Since then, scientists have made continuous efforts to extend the light reactivity of TiO2 photocatalysts into the visible part of the spectrum.
Bacteriorhodopsin—which is responsible for the unusual purple color of a number of salt flats in California and Nevada—uses sunlight as an energy source that allows it to act as a proton pump. Proton pumps are proteins that typically straddle a cellular membrane and transfer protons from inside the cell to the extracellular space. In this study, the protons provided by the bR are combined with free electrons at small platinum sites interspersed in the TiO2 matrix. This bio-assisted hybrid photocatalyst outperforms many other similar systems in hydrogen generation and could be a good candidate for fabrication of green energy devices that consume virtually infinite sources—saltwater and sunlight.
More information: Balasubramanian, S. et al. Nano Lett., 13, 3365 (2013).
Journal information: Nano Letters
Provided by Argonne National Laboratory