For better batteries, just add water
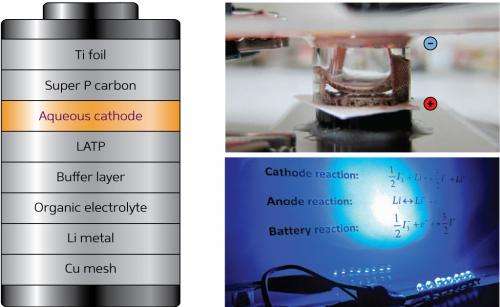
Lithium-ion batteries are now found everywhere in devices such as cellular phones and laptop computers, where they perform well. In automotive applications, however, engineers face the challenge of squeezing enough lithium-ion batteries onto a vehicle to provide the desired power and range without introducing storage and weight issues. Hye Ryung Byon, Yu Zhao and Lina Wang from the RIKEN Byon Initiative Research Unit have now developed a lithium-iodine battery system with twice the energy density of conventional lithium-ion batteries.
Byon's team is involved in alternative energy research and, specifically, improving the performance of lithium-based battery technologies. In their research they turned to an 'aqueous' system in which the organic electrolyte in conventional lithium-ion cells is replaced with water. Such aqueous lithium battery technologies have gained attention among alternative energy researchers because of their greatly reduced fire risk and environmental hazard. Aqueous solutions also have other advantages, which include an inherently high ionic conductivity.
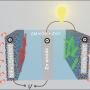
For their battery system, the researchers investigated an 'aqueous cathode' configuration (Fig. 1), which accelerates reduction and oxidation reactions to improve battery performance. Finding suitable reagents for the aqueous cathode, however, proved to be a tricky proposition. According to Byon, water solubility is the most important criterion for screening new materials, since this parameter determines the battery's energy density. Furthermore, the redox reaction has to take place in a restricted voltage range in order to avoid water electrolysis. An extensive search led the researchers to produce the first-ever lithium battery involving aqueous iodine—an element with high water solubility and a pair of ions, known as the triiodide/iodide redox couple, that readily undergo aqueous electrochemical reactions.
The team constructed a prototype aqueous cathode device and found the energy density to be nearly double that of a conventional lithium-ion battery, thanks to the high solubility of the triiodide/iodide ions. Their battery had high and near-ideal power storage capacities and could be successfully recharged hundreds of times, avoiding a problem that plagues other alternative high-energy-density lithium-ion batteries. Microscopy analysis revealed that the cathode collector remained untouched after 100 charge/discharge cycles with no observable corrosion or precipitate formation.
Byon and colleagues now plan to develop a three-dimensional, microstructured current collector that could enhance the diffusion-controlled triiodide/iodide process and accelerate charge and discharge. They are also seeking to raise energy densities even further by using a flowing-electrode configuration that stores aqueous 'fuel' in an external reservoir—a modification that should make this low-cost, heavy metal-free design more amenable to electric vehicle specifications.
More information: 1.Zhao, Y., Wang, L. & Byon, H. R. High-performance rechargeable lithium-iodine batteries using triiodide/iodide redox couples in an aqueous cathode. Nature Communications 4, 1896 (2013). dx.doi.org/10.1038/ncomms2907
Journal information: Nature Communications
Provided by RIKEN