Team develops new technique to track cell interactions in living bodies
Researchers at Stanford University School of Medicine have developed a new technique to see how different types of cells interact in a living mouse. The process uses light-emitting proteins that glow when two types of cells come close together.
Using the technique, the team was able to pinpoint where in the body metastatic cancer cells ended up after they broke off from an initial tumor site, using readily available lab reagents. The team chose chemicals that are easily available in most life sciences laboratories because they wanted to develop a technique that could be widely used.
The study will be published online May 6 in the Proceedings of the National Academy of Sciences.
Until now, the best way to see cells interacting inside a living animal or person was to implant a microscope. But predicting all the places metastatic cancer cells will proliferate is nearly impossible. "There are currently no great ways to look at early metastasis, where metastases are finding their micro-environments and setting up shop," said Mark Sellmyer, MD, PhD, the study's lead author, who developed the technique as a graduate student working jointly for Christopher Contag, PhD, professor of pediatrics and of microbiology and immunology, and Tom Wandless, PhD, associate professor of chemical and systems biology.
Sellmyer, who begins a research residency in radiology at the University of Pennsylvania this summer, worked closely with Jennifer Prescher, PhD, a former postdoctoral scholar in Contag's lab who is now an assistant professor at the University of California-Irvine. "I think it really expands our capabilities, expands the tool box," Prescher said. "We're always beholden to the tools available for us in terms of what we can observe. Any time we have a new vantage point - a new technology - it can open doors to understanding new aspects of biology."
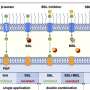
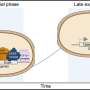
Sellmyer and Prescher genetically altered immune cells and cancer cells - what they call activator and reporter cells, respectively - so the cells would produce two different enzymes. The activator immune cells created the enzyme B-galactosidase, which can convert a common biological probe called a "caged luciferin" into luciferin, a molecule naturally found in animals like fireflies. The reporter cancer cells created the enzyme luciferase, which splits the luciferin molecule in a chemical reaction that emits light.
After demonstrating the process could work in a cell culture in a petri dish, they used the same method in living mice. The mice were implanted with genetically altered bone marrow cells that generated activator immune cells. An experimental group of 10 mice had tumors made of cancer cells genetically altered to serve as reporters implanted into their abdomens. For comparison, a control group of four additional mice were implanted with unaltered bone marrow cells. After several weeks, the researchers injected a compound that would be converted to light-emitting luciferin.
The researchers could easily see where populations of immune cells got close to metastatic cancer cell populations because those parts of the mouse body would glow and take a picture of the pattern of light emitted by the mouse's body. Among other metastasis sites, researchers were surprised to find that in several mice, small populations of cancer cells had started to grow in their noses and just under their lower jaws.
Currently, the technique can't be used in people, but the researchers hope it will be used to study a variety of cell interactions in laboratory mice. Contag is planning to use the technique to study how immune cells migrate to sites of infection. Cell-cell communication is important for a variety of biological processes," he said. "Knowing the proximity of one cell type to another in the context of the living tissue is key for understanding biology."
The technique requires a minimum population of 1,000 cells for both the activator and reporter cells to work. Prescher is working on improving the precision of the method so that smaller populations with fewer cells can be tracked.
Both Contag and Wandless noted that Sellmyer's and Prescher's interdisciplinary approach was key to developing the technique. "Their training in biology allowed them to identify an important problem in the area of in vivo imaging, and their ability to incorporate chemistry allowed Mark and Jenn to design and develop a new technique that would be out-of-reach to the majority of classical biology labs," Wandless said.
More information: Visualizing cellular interactions with a generalized proximity reporter, www.pnas.org/cgi/doi/10.1073/pnas.1218336110
Journal information: Proceedings of the National Academy of Sciences
Provided by Stanford University Medical Center