Researchers propose new mechanism for cell membrane fission
A study led by the Membrane Nanomechanics group of the Biophysics Unit of the UPV/EHU-University of the Basque Country has made it possible to characterise the functioning of a protein responsible for cell membrane splitting. The results of the study, published in the prestigious journal Science, make it possible to see the basic mechanisms of cell life from a fresh perspective, like the fusion and splitting of cell membranes. What is more, the methodology developed will allow various neuromuscular disorders to be diagnosed.
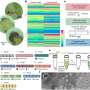
Cells have a series of specialised proteins so that their membranes can join together (fuse) or separate (split) without losing their protective role against the external medium. One of these specialised proteins is the protein dynamin responsible for the constriction and fission of the necks of endocytic vesicles. Two of the main characteristics of dynamin are its assembly capacity on membranes with high curvature (the necks of the vesicles) and its GTP activity, in other words, the capacity to use the energy stored inside the GTP molecules. GTP, which stands for guanosine triphosphate, is a chemical compound that plays a crucial role in cell metabolism.
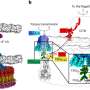
It was hitherto thought that the dynamin used the GTP energy to produce a very strong constriction of the neck of the vesicle and thus bring about its fission. Nevertheless, the study led by the Ikerbasque lecturer, Vadim Frolov, has enabled the fission action by the dynamin to be characterised for the first time on nanometric scales and with great time resolution. "We have managed to characterise the minimal functional unit of the dynamin," says the researcher. This study has made it possible to separate the membrane splitting process by the dynamin into two stages: the first, a purely mechanical one in which the constriction of the vesicle neck takes place, and the second, in which the dynamin "functions like a catalytic centre by inserting some of its domains into the membrane," explains Frolov. "The GTP hydrolysis increases the internal flexibility of the dynamin molecule, thus allowing the optimum shape of the protein to be found on the membrane so that it splits. This optimization constitutes the essence of "geometric catalysis", a new way of seeing the activity of the proteins while the membrane is being remodelled," he adds.
Protein involved in neurodegenerative diseases
According to Frolov, this study has marked "the start of a new line of research in the Membrane Nanomechanics group." In fact, this project, which has had a two-year duration, has led to "the specification and development of the method necessary to be able to characterise the action of dynamin with great space-time precision." It is a combination of fluorescence microscopy measurements and electrophysiological ones. "Now we are in a position to measure the passing of the ions along the inside of a lipid nanotube while we observe it by means of fluorescence microscopy. The result can be translated into a technique that allows very fast processes on very small scales to be characterised," says Frolov.
"This technique will enable us to study why small mutations in the dynamin lead to various human diseases, like neuromuscular diseases," adds Frolov.
Journal information: Science
Provided by Universidad del Pais Vasco