X-ray laser breakthrough research shows chemical reaction in real time
The ultrafast, ultrabright X-ray pulses of the Linac Coherent Light Source (LCLS) have enabled unprecedented views of a catalyst in action, an important step in the effort to develop cleaner and more efficient energy sources.
Scientists at the U.S. Department of Energy's (DOE) SLAC National Accelerator Laboratory used LCLS, together with computerized simulations, to reveal surprising details of a short-lived early state in a chemical reaction occurring at the surface of a catalyst sample. The study offers important clues about how catalysts work and launches a new era in probing surface chemistry as it happens.
"To study a reaction like this in real time is a chemist's dream," said Anders Nilsson, deputy director for the Stanford and SLAC SUNCAT Center for Interface Science and Catalysis and a leading author in the research, published Mar. 15 in Science. "We are really jumping into the unknown."
Catalysts, which can speed up chemical reactions and make them more efficient and effective, are essential to most industrial processes and to the production of many chemicals. Catalytic converters in cars for example, reduce emissions by converting exhaust to less toxic compounds. Understanding how catalysts work, at ultrafast time scales and with molecular precision, is essential to producing new, lower-cost synthetic fuels and alternative energy sources that reduce pollution, Nilsson said.
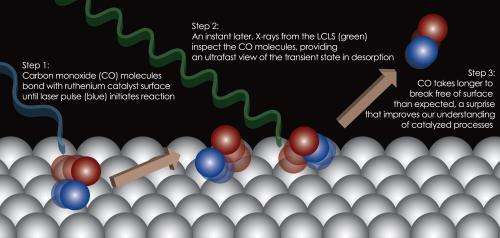
In the LCLS experiment, researchers looked at a simple reaction in a crystal composed of ruthenium, a catalyst that has been extensively studied, in reaction with carbon monoxide gas. The scientists zapped the crystal's surface with a conventional laser, which caused carbon monoxide molecules to begin to break away. They then probed this state of the reaction using X-ray laser pulses, and observed that the molecules were temporarily trapped in a near-gas state and still interacting with the catalyst.
"We never expected to see this state," Nilsson said. "It was a surprise."
Not only was the experiment the first to confirm the details of this early stage of the reaction, it also found an unexpectedly high share of molecules trapped in this state for far longer than what was anticipated, raising new questions about the atomic-scale interplay of chemicals that will be explored in future research.
Some of the early stages of a chemical reaction are so rapid that they could not be observed until the creation of free-electron lasers such as LCLS, said Jens Nørskov, director for SUNCAT. Future experiments at LCLS will examine more complex reactions and materials, Nilsson said. "There is potential to probe a number of catalytic-relevant processes – you can imagine there are tons of things we could do from here."
Important preliminary research was conducted at SLAC's Stanford Synchrotron Radiation Lightsource (SSRL), and this direct coupling of research at SLAC's synchrotron and X-ray laser proved essential, said Hirohito Ogasawara, a staff scientist at SSRL.
More information: "Real-Time Observation of Surface Bond Breaking with an X-ray Laser"; Martina Dell´Angela et al.; Science, 2013; DOI: 10.1126/science.1231711
Journal information: Science
Provided by SLAC National Accelerator Laboratory