February 8, 2013 weblog
Researchers create reusable adhesive that liquefies under UV light
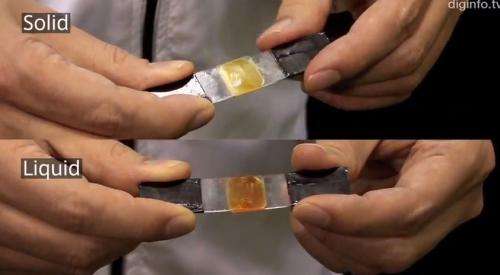
(Phys.org)—Researchers at Japan's National Institute of Advanced Industrial Science and Technology, (AIRT) have developed an organic material that can be liquefied or hardened by shining different types of light on it. The result is an adhesive that can be used over and over again.
Engineers from AIRT demonstrated the material at NanoTech 2013, currently running in Tokyo. The material was first presented as a powdered solid. Shining UV light on it causes it to liquefy where it can be applied to a surface. Once in place, shining a green light on it causes it to become hard, bonding two pieces of material together. In the demonstration, two small glass plates were "glued" together using nothing but the material and UV light—they were subsequently "unglued" by shining a green light at the point of connection. In addition to changing between liquid and solid states, the material also changes color. It's yellow as a solid, but turns orange as it liquefies.
The AIRT representative at the show noted that the material does not undergo a reaction where new bonds form and are broken as it moves between a liquid and solid state, instead, an isomerization reaction occurs where the shape of the material itself changes. Because of that, the material can be used over and over again without losing its characteristics. They also noted that the speed of the reaction varies depending on the amount of light applied. Also, to cause the material to liquefy, the light used must be in the range 365 nm to 385 nm. To cause it to harden, the material can be exposed to ordinary light, as it contains light in the green range. This is the slow way, they say—using a green light, speeds up the process dramatically—a film 10 microns thick, for example, would take about 2 minutes to harden under a 80mW light source. It's important to note, they add, that the change occurs due to light only—no heat is required to cause it to come about. They add that the adherence strength of the material is roughly equivalent to double-sided tape.
AIRT believes the new material might be useful in manufacturing applications, where parts need to be held together temporarily while work is done, than released. Other applications might be more difficult to implement due to the necessity of working with transparent materials that allow light to pass through to the adhesive. Company reps say their engineers are continuing to study the material to see if its adhesive strength might by increased, and to find a way to change its color.
More information: via Diginfo.tv
© 2013 Phys.org




















