January 24, 2013 feature
Nanosilicon rapidly splits water without light, heat, or electricity
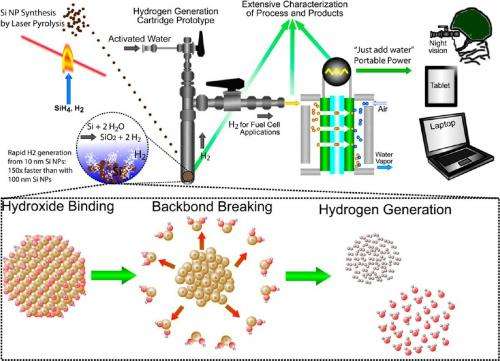
(Phys.org)—Although scientists know that when silicon mixes with water, hydrogen is produced through oxidation, no one expected how quickly silicon nanoparticles might perform this task. As a new study has revealed, 10-nm silicon nanoparticles can generate hydrogen 150 times faster than 100-nm silicon nanoparticles, and 1,000 times faster than bulk silicon. The discovery could pave the way toward rapid "just add water" hydrogen generation technologies for portable devices without the need for light, heat, or electricity.
The researchers, Folarin Erogbogbo at the University of Buffalo and coauthors, have published their paper on using nanosilicon to generate hydrogen in a recent issue of Nano Letters.
If hydrogen is ever to be used to deliver energy for wide commercial applications, one of the requirements is finding a fast, inexpensive way to produce hydrogen. One of the most common hydrogen production techniques is splitting water into hydrogen and oxygen. There are several ways to split water, such as with an electric current (electrolysis), heat, sunlight, or a substance that chemically reacts with water. Such substances include aluminum, zinc, and silicon.
As the scientists explained, silicon-water oxidation reactions have so far been slow and uncompetitive with other water splitting techniques. However, silicon does have some theoretical benefits, such as being abundant, being easy to transport, and having a high energy density. Further, upon oxidation with water, silicon can theoretically release two moles of hydrogen per mole of silicon, or 14% of its own mass in hydrogen.
For these reasons, the scientists decided to take a closer look at silicon, specifically silicon nanoparticles, which have not previously been studied for hydrogen generation. Because silicon nanoparticles have a larger surface area than larger particles or bulk silicon, it would be expected that the nanoparticles can generate hydrogen more rapidly than the larger pieces of silicon.
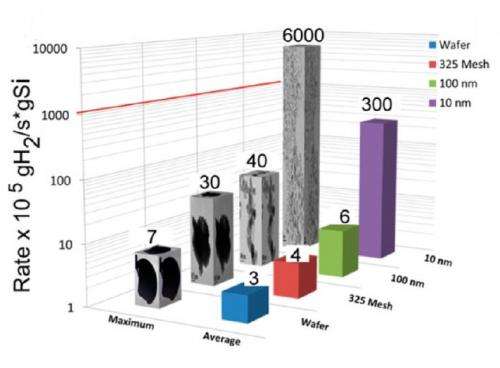
But the improvements the scientists discovered with silicon nanoparticles far exceeded their expectations. The reaction of 10-nm silicon particles with water produced a total of 2.58 mol of hydrogen per mol of silicon (even exceeding theoretical expectations), taking 5 seconds to produce 1 mmol of hydrogen. In comparison, the reaction with 100-nm silicon particles produced a total of 1.25 mol of hydrogen per mole of silicon, taking 811 seconds to produce each mmol of hydrogen. For bulk silicon, total production was only 1.03 mol of hydrogen per mol of silicon, taking a full 12.5 hours to produce each mmol of hydrogen. For a rate comparison, the 10-nm silicon generated hydrogen 150 times faster than 100-nm silicon and 1,000 times faster than bulk silicon.
"I believe the greatest significance of this work is the demonstration that silicon can react with water rapidly enough to be of practical use for on-demand hydrogen generation," coauthor Mark Swihart, Professor of Chemical and Biological Engineering at the University of Buffalo, told Phys.org. "This result was both unexpected and of potential practical importance. While I do not believe that oxidation of silicon nanoparticles will become a feasible method for large-scale hydrogen generation any time soon, this process could be quite interesting for small-scale portable applications where water is available."
In addition to producing hydrogen faster than larger silicon pieces, the 10-nm silicon also produces hydrogen significantly faster than aluminum and zinc nanoparticles. As Swihart explained, the explanation for this inequality differs for the two materials.
"Compared to aluminum, silicon reacts faster because aluminum forms a denser and more robust oxide (Al2O3) on its surface, which limits the reaction," he said. "In the presence of a base like KOH [potassium hydroxide], silicon mostly produces soluble silicic acid (Si(OH)4). Compared to zinc, silicon is simply more reactive, especially at room temperature."
Although the larger surface area of the 10-nm silicon compared with larger silicon pieces contributes to its fast hydrogen production rate, surface area alone cannot account for the huge rate increase that the scientists observed. The surface area of 10-nm silicon is 204 m2/g, about 6 times greater than the surface area of 100-nm silicon, which is 32 m2/g.
To understand what causes the much larger increase in the hydrogen production rate, the researchers conducted experiments during the silicon etching process. They found that, for the 10-nm particles, etching involves the removal of an equal number of lattice planes in each direction (isotropic etching). In contrast, for 100-nm particles and microparticles, unequal numbers of lattice planes are removed in each direction (anisotropic etching).
The researchers attribute this etching difference to the different geometries of different-sized crystals. As a result of this difference, the larger particles adopt non-spherical shapes that expose less reactive surfaces compared to the smaller particles, which remain nearly spherical, exposing all crystal facets for reaction. Larger particles also develop thicker layers of oxidized silicon byproducts through which water must diffuse. Both of these factors limit the rate of the reaction on larger particles.
To confirm that that the 10-nm silicon-water reaction generates hydrogen with no byproducts that could interfere with applications, the researchers used the silicon-generated hydrogen to operate a fuel cell. The fuel cell performed very well, producing more current and voltage than the theoretical amount of pure hydrogen, which is due to the fact that the 10-nm particles generated more hydrogen than the theoretical 14 wt %.
The researchers hope that this surprising ability of silicon nanoparticles to rapidly split water and generate hydrogen could lead to the development of a hydrogen-on-demand technology that could enable fuel cells to be used in portable devices. This technology would require a large-scale, energy-efficient method of silicon nanoparticle production, but could have some advantages compared to other hydrogen generation techniques.
"The key advantage of silicon oxidation for hydrogen generation is its simplicity," Swihart said. "With this approach, hydrogen is produced rapidly, at room temperature, and without the need for any external energy source. The energy needed for hydrogen generation is effectively stored in the silicon. All of the energy input required for producing the silicon can be provided at a central location, and the silicon can then be used in portable applications.
"The key disadvantage of silicon oxidation is its relative inefficiency. The energy input required to create the silicon nanoparticles is much greater than the energy available from the hydrogen that is finally produced. For large scale applications, this would be a problem. For portable applications, it is not. For example, the cost of electricity supplied by an ordinary household battery can easily be 10 to 100 times higher than the cost of electricity from a utility, but batteries still play an important role in our lives."
In the future, the researchers plan to further increase the hydrogen generation capacity of silicon oxidation by experimenting with different mixtures.
"One direction that we are presently pursuing is the use of mixtures of silicon nanoparticles with metal hydrides, which also react with water to produce hydrogen," Swihart said. "Compounds like lithium hydride and sodium hydride react with water to produce the base (LiOH or NaOH) that is needed to catalyze the silicon oxidation. However, they can react too fast with water (explosively) and are not stable in air. Mixing them with silicon nanoparticles or coating them with silicon nanoparticles may serve to both temper their reactivity and increase the hydrogen generation capacity of the system by replacing the added base (e.g., KOH in the published paper) with a material that also generates hydrogen."
More information: Folarin Erogbogbo, et al. "On-Demand Hydrogen Generation using Nanosilicon: Splitting Water without Light, Heat, or Electricity." Nano Letters. DOI: 10.1021/nl304680w
Journal information: Nano Letters
Copyright 2013 Phys.org
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of Phys.org.