'Comet water' ions found in bacterial protein
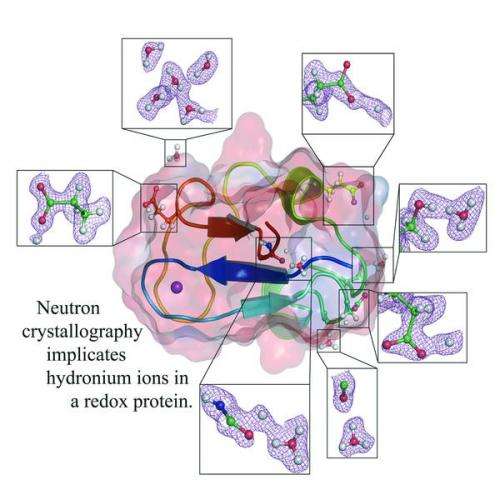
Developments arising from new science techniques at Keele University in the UK, the Institut Laue-Langevin (ILL), the flagship centre for neutron science, and the European Synchrotron Radiation Facility (ESRF), have confirmed the presence of hydronium ions in the protein rubredoxin. Rubredoxin is a light weight iron-sulphur protein found in some of the earliest, most basic forms of life, notably bacteria and archaea. These ions, commonly found in comet tails or interstellar space clouds, have been found to be involved in crucial interactions with the protein.
The new results, reported in Angewandte Chemie, combine the use of one of the world's most sophisticated diffractometers with a novel sample preparation process whereby the protein's hydrogen atoms are replaced with the heavier isotope, deuterium, greatly enhancing the visualisation of hydronium ions.
A new tool for neutron science
Despite their clearly particle-like character, neutrons demonstrate wave like behaviour; when they encounter obstacles whose size is comparable with their wavelength, they scatter in such a way that allows scientists to determine the structure of the material through which the neutrons have passed. Neutrons are particularly good in studying biological materials since they are highly sensitive to lighter atoms such as hydrogen - strongly complementing the capabilities of synchrotron X-rays which are more sensitive to heavier elements.
However in the past neutron scientists have run into difficulties when studying proteins due to excessive levels of background noise in the measured data, drastically limiting the scope of analysis work. This problem can be totally avoided by replacing the hydrogen atoms in the protein by deuterium. In response, a team of scientists at Keele University (Macromolecular Structure Group) in the UK and at the ILL (Life Sciences Group), with the aid of funding from the UK Engineering and Physical Sciences Research Council (EPSRC). have developed a unique Deuteration Laboratory that allows biological macromolecules to be deuterated in a way that optimises the quality of the data collection and of the final results.
This study was carried out through a collaboration involving Keele, the ILL, and the ESRF. Deuterated rubredoxin was produced and the scattering (diffraction) experiments carried out on the ILL's world leading monochromatic neutron beamline D19. Uniquely amongst neutron diffraction instruments, the detector on D19 has a curved 'banana' shape that covers a wide angle around the sample allowing the acquisition of remarkably high-quality data.
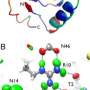
Rubredoxin occurs in two forms, or oxidation states, and moves between them by accepting or losing electrons from other molecules in the organism in what are known as redox reactions. These reactions play a critical role in vital biological processes such as respiration, photosynthesis, and nitrogen fixation. An important aspect of this work was therefore to elucidate changes in the protein molecule that could provide an understanding of the way redox mechanisms work – and the role played by hydrogen atoms and water.
The study provided a major advance well beyond anything achieved previously, and has opened up a new and extremely important area of protein science. The team characterised both the reduced and oxidised forms of the protein at near-atomic resolution. Surprisingly, the results showed the presence of numerous hydronium (H3O+) ions within the protein. Whilst H3O+ ions have been identified in chemical systems and in one protein, this is the first time they have been found in a redox protein where they are likely to be deeply implicated in charge transfer.
Furthermore the study demonstrates shifts of hydrogen atoms in relation to hydronium ions following the change between the reduced and oxidised forms of the protein. Dr Maxime Cuypers from Keele University and the Institut Laue-Langevin said: "This work started off as a proof of concept study to understand and exploit the potential of this new combination of techniques. However when we analysed the data it rapidly became obvious that there was a lot more going on than we could have predicted. What we have here is a remarkable set of results that not only demonstrates the unique power of high-resolution monochromatic neutron crystallography and also the use of deuterated proteins to probe protein structures, but also the opening of a totally new area of protein science that is directly relevant to critical aspects of biological function."
More information: onlinelibrary.wiley.com/doi/10 … /anie.201207071/full
Journal information: Angewandte Chemie
Provided by Institut Laue-Langevin