Silver sheds light on superconductor secrets
(Phys.org)—By doping a bismuth-based layered material with silver, Chinese scientists demonstrated that superconductivity is intrinsic to the new material rather than stemming from its impurities.
The first report on the chemical substitution, or doping, using silver atoms, for a new class of superconductor that was only discovered this year, is about to be published in European Physical Journal B. Chinese scientists from Institute of Solid State Physics, Chinese Academy of Sciences, Hefei, discovered that the superconductivity is intrinsic rather than created by impurities in this material with a sandwich-style layered structure made of bismuth oxysulphide (Bi4O4S3).
Superconductors with a transition temperature (TC) above the boiling temperature of liquid nitrogen (77 kelvins or −196 °C) are called high TC superconductors. In the quest for such materials, compounds with bismuth disulphide (BiS2) layers have recently started to attract a lot of attention. Indeed, in July 2012, Japanese scientists reported achieving a TC at around 4.5 kelvins (-268.65 °C) with the first bismuth oxysulphide superconductor.
All the superconducting samples for this new superconductor reported so far are a mixture of Bi4O4S3 and impurities. However, the pure sample without impurities is not superconducting. Scientists have therefore been wondering whether the observed superconductivity stems from the presence of impurities.
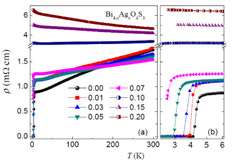
The Hefei team performed systematic measurement of the material's characteristics relying on x-ray diffraction, magnetic susceptibility, electrical transport and thermal transport. Using comparison of the x-ray diffraction patterns, they found that silver atoms partially replace the bismuth sites in the bismuth oxysulphide lattice.
Further experiments involved controlling the composition of the material through various levels of silver doping. The superconductivity, the authors found, was suppressed as the silver content increases and eventually disappears above a certain doping threshold. They believe that it is the modification of electronic structure upon doping that suppresses the superconductivity. Based on these observations, they concluded that the observed superconductivity originates from the bismuth oxysulphide lattice rather than any impurities.
More information: 1. S. G. Tan, P. Tong, Y. Liu, W. J. Lu, L. J. Li, B. C. Zhao, Y. P. Sun (2012), Suppression of superconductivity in layered Bi4O4S3 by Ag doping, European Physical Journal B, DOI: 10.1140/epjb/e2012-30975-2
2. Mizuguchi et al. arXiv:1207.3145 [cond-mat.supr-con]
Journal information: European Physical Journal B
Provided by Springer