Researchers discover a new type of toxic protein, reveal its 3-D structure
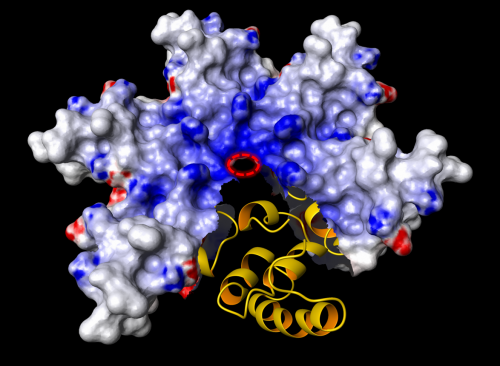
(Phys.org)—Researchers at Kiel University have discovered a toxic protein of pathogenic acanthamoebae and have been able to elucidate its three-dimensional structure. They found that this protein molecule looks different from all the structures formerly discovered. The acanthamoeba secretes a cell toxin (Acanthaporin); when roused from its inactive state this toxin infiltrates human nerve cells or bacteria and embeds itself in the plasma membrane, forming a kind of circular channel (pore). This discovery is published in the current issue of Nature Chemical Biology.
Acanthamoebae are free-living unicellular organisms, which occur in soil and water where they feed on other microorganisms. For humans, certain amoebas of this genus are dangerous because they can cause diseases. The parasite can infest the nervous system and may cause death. Compared to commonly known tropical diseases such as malaria or amoebic dysentery, which are also caused by parasitic unicellular organisms, such a fatal outcome is relatively rare and affects primarily patients with a weak immune system. In the medical field, acanthamoeba is better known as an actuator for painful inflammation of the cornea, known as amoebic keratitis. People who wear contact lenses mainly suffer from this disease that can lead to complete blindness. Furthermore, the parasite often carries other bacterial pathogens within its cellular body like the one causing Legionnaire's disease. For this reason, acanthamoebae are dubbed "Trojan horses".
The current scientific findings are the result of close collaboration between two teams of scientists supervised by Professor Matthias Leippe (Zoophysiology, Institute of Zoology at Kiel University) und Professor Joachim Grötzinger (Structural Biology, Institute of Biochemistry at Kiel University). The findings were to a major extent collected by Matthias Michalek, then a mutual doctorate candidate studying under both Leippe and Grötzinger. As the project started to grow, the scientists were joined by colleagues from Kiel University and from the Research Center Borstel. The latter has been partner to Kiel University in the cluster "Inflammation at Interfaces" for some years. Furthermore, international experts were asked to join the research.
"For scientists like us, who work on the fundamentals, it is very satisfying if we are able not only to discover such an interesting protein, but in the end are able to see exactly what it looks like. In that way we can learn more about its mode of action", said the initiator of the study, Matthias Leippe, adding: "It is even more satisfying if it turns out to be a completely new structure. A discovery such as this hardly ever happens nowadays." He continued to explain: "The principle of distributing soluble proteins which incorporate themselves into cell membranes and form pores is however not new. We find the same mechanisms with bacterial pathogens or in our own immune system. This is why we refer to these proteins as 'ancient weapons' that take effect very fast and very effectively. In nature, these mechanisms have proven to be successful for attack and defence."
The scientists hope that thanks to their findings, it will be easier to understand the mysterious circumstances which cause tissue damage and disease caused by acanthamoeba.
More information: The original article was published here: "Structure and function of a unique pore-forming protein from a pathogenic acanthamoeba"; DOI:10.1038/NChemBio.1116
Journal information: Nature Chemical Biology
Provided by Kiel University