Nanoparticles improve lithium battery electrodes
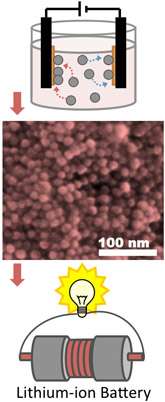
(Phys.org)—Materials scientists have developed a simple, robust way to fabricate carbon-free and polymer-free, lightweight colloidal films for lithium-ion battery electrodes, which could greatly improve battery performance.
By developing a method for additive-free electrodes that maintain high conductivity, the researchers have opened new possibilities for reducing the weight and volume of batteries, while also creating a template system for studying the physics of nanoparticle electrodes.
The work, led by Richard Robinson, assistant professor of materials science and engineering, and graduate student Don-Hyung Ha, is featured in the Oct. 10 issue of Nano Letters (Vol. 12, No. 10).
Nanoparticles have been extensively investigated as an active cathode and anode in lithium-ion batteries—common components of electronic devices—because they can enhance the batteries' electrochemical properties.
To use colloidal nanoparticles for the electrodes, it had been necessary to combine them with carbon-based conductive materials for enhancing charge transport, as well as polymeric binders to stick the particles together and to the electrode substrate, Robinson said. This process added extra weight to the battery and made it difficult to model the movement of Li-ions and electrons through the mixture.
The critical processing technique Robinson and colleagues used was electrophoretic deposition, which binds the metal nanoparticles to the surface of the electrode substrate to each other in an assembly, creating strong electrical contacts between the particles and current collector.
The process results in a significant improvement in battery electrode assembly that cannot be replicated by conventional methods. Once attached, the particles are no longer soluble and are mechanically robust. In fact, this processing creates a film that has superior mechanical stability when compared to films fabricated by conventional battery-making methods with binders, Robinson said.
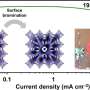
This research has led to the first cobalt-oxide nanoparticle-film battery electrode made without using binders and carbon black additives, and they show high gravimetric and volumetric capacities, even after 50 cycles.
Journal information: Nano Letters
Provided by Cornell University