3D structure of an unmodified G protein-coupled receptor in its natural habitat
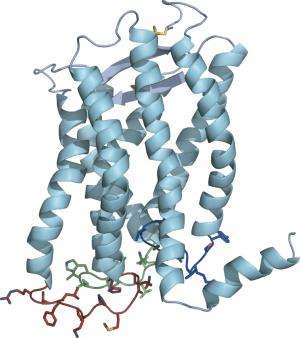
Scientists have determined the three-dimensional structure of a complete, unmodified G-protein-coupled receptor in its native environment: embedded in a membrane in physiological conditions.
Using NMR spectroscopy, the team mapped the arrangement of atoms in a protein called CXCR1, which detects the inflammatory signal interleukin 8 and, through a G protein located inside the cell, triggers a cascade of events that can mobilize immune cells, for example.
Because G protein-coupled receptors are critical for many cellular responses to external signals, they have been a major target for drugs. More precise knowledge of the shapes of these receptors will allow drugmakers to tailor small molecules to better fit specific targets, avoiding collateral hits that can cause detrimental side effects.
"This finding will have a major impact on structure-based drug development since for the first time the principal class of drug receptors can be studied in their biologically active forms where they interact with other proteins and potential drugs," said Stanley Opella, professor of chemistry and biochemistry at the University of California, San Diego who led the work, which Nature published online October 21st in advance of the print edition.
Protein structures are most often determined by reading the diffraction patterns of X-rays beamed at their crystalline form, but crystallizing such large, unwieldy molecules is a challenge often met with strategies such as snipping off floppy ends.
Those changes can alter the shape of critical regions of the protein. "Our approach was to not touch the protein," Opella said. "We are working with molecules in their active form."
Their strategy has revealed a new view of these receptors. Previous reports have all noted seven helices weaving through the membrane. Opella's group sees an eighth lying on the membrane surface, a trait that at least some other G protein-coupled receptors must share.
And the loops inside and outside of the cell are structured. "For years people thought the loops were mobile. They're not," Opella said. "The signals we get from the loops aren't any weaker than the other parts of the protein as they would be if they were waving about."
CXCR1 has been implicated in the progression of several types of cancer. In one example, preclinical studies have shown that blocking this receptor inhibits the undifferentiated stem cells within breast cancer tumors, leading to the death of all tumor cell types and stopping them from seeding new tumors.
Opella and colleagues hope this finding along with continuing studies of changes in this receptor's configuration as it binds to interleukin 8 and drug candidate will lead to more effective and less harmful cancer treatments.
Journal information: Nature
Provided by University of California - San Diego


















