No more bubbles when boiling water
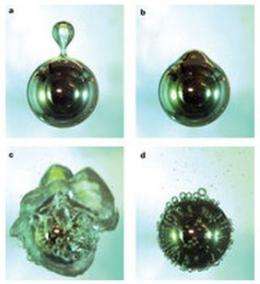
(Phys.org)—The research, which is the first of its kind, has identified a specially engineered steel surface that allows liquids to boil without bubbling.
"This would be advantageous for use in industrial situations such as nuclear power plants, where vapour explosions are best avoided, or where gentle heating is desirable" said Professor Derek Chan, from the University's Department of Mathematics and Statistics.
The study suggests that the new surface could also be applied to other situations that involve the transfer of heat, such as reducing fogging and preventing ice or frost formation on windows.
"Our results show the potential of using this textured surface to control heating and cooling events that affect the formation of frost on windows and ice on the control surfaces of aircrafts or even refrigeration units," he said.
The international study was done in collaboration between the University of Melbourne and Dr Neelesh Patankar from the Northwestern University in the United States and Dr Ivan Vakarelski and his team at the King Abdullah University of Science and Technology in Saudi Arabia where the experimental studies were carried out.
The study was published today in the journal Nature.
The research found that a textured, highly water-repellent steel surface controls the boiling process of a liquid and stops it from bubbling up the sides of a container and boiling over.
This is achieved by using a textured surface structure to control the stability of the vapour layer, that is, the layer of steam that forms on a surface when water is boiled.
"In most smooth surfaces, heat transfer from the surface to the liquid is prevented by the low thermal conductivity of the vapour layer," said Professor Chan.
"This vapour layer collapses when the surface cools, which could result in an explosion."
Professor Chan said that in textured surfaces, the vapour layer is maintained until the surface is completely cooled, preventing the liquid from bubbling and boiling over.
"The discovery shows how the texture of surfaces can combine to control the boiling of liquid in a way that was not thought to be possible", he said.
Journal information: Nature
Provided by University of Melbourne



















