July 17, 2012 feature
Artificial photosynthesis system gets efficiency boost from graphene
(Phys.org) -- By converting sunlight into chemical energy, artificial photosynthesis systems could potentially produce renewable, nonpolluting fuels and chemicals for a wide variety of uses. But developing an efficient solar-to-fuel conversion process has turned out to be extremely challenging. Although researchers have demonstrated the feasibility of artificial photosynthesis, achieving a high efficiency has proven to be more difficult.
In a new study, a team of scientists from the Korea Research Institute of Chemical Technology in Daejon, South Korea, and Ewha Womans University in Seoul, South Korea, have demonstrated that graphene may serve as an efficient photocatalyst for improving the efficiency of an artificial photosynthesis system. As a photocatlyst, graphene uses sunlight to spur the reaction without becoming involved itself.
As the researchers explain, a good photocatalyst for such a system should operate in the visible light spectrum, since 46% of total sunlight energy on Earth is in the visible range and only 4% in the UV range. Previous studies have experimented with graphene-semiconductor composites as photocatalysts, but the results showed that these materials had low electron transfer levels, which led to low efficiencies.
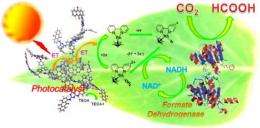
In the new study, the scientists used graphene by itself as the photocatalyst, which they then coupled to a porphyrin enzyme. The researchers demonstrated that this material could convert sunlight and carbon dioxide into formic acid, a chemical that is used in the plastic industry and as fuel in fuel cells. Tests showed that the graphene-based photocatalyst is highly functional in the visible light regime, and that its overall efficiency is significantly higher than the efficiencies of other photocatalysts.
“The photocatalyst-enzyme coupled system is one of the most ideal artificial photosynthesis systems that utilize solar energy for the synthesis of various chemicals and fuel,” coauthor Jin-Ook Baeg at the Korea Research Institute of Chemical Technology told Phys.org. “For practical use of the photo-bioreactor artificial photosynthesis process, one of the most challenging tasks is the search for a highly efficient visible light active material which acts as a photocatalyst in the NADH regeneration system and triggers enzymatic production of solar chemicals/solar fuel from CO2. As a step towards this goal, we report the synthesis of a novel graphene-based visible light active material as a photocatalyst of the photo-bioreactor system for an efficient artificial photosynthetic production of formic acid from CO2.”
To understand the origins of the enhanced photocatalytic activity, the researchers examined the photocatalyst using spectroscopy, thermal analysis, and microscopy techniques. They found that the material has good electron transfer abilities, and graphene’s large surface area helps accelerate the chemical reactions in the conversion process.
The ability to produce solar fuel directly from CO2 has applications not only for fuel cells and plastics, but also in the pharmaceutical industry.
“As one of the strong practical merits of the system, it also can be used for the production of tailor-made fine chemicals using solar light energy,” Baeg said. “For example, chiral 2-amino-1-arylethanol derivatives are a very important intermediate of many kinds of very expensive chiral drugs. It can be easily synthesized by our photocatalyst-enzyme coupled artificial photosynthesis system simply using solar light energy. We are presently doing the research to develop the photocatalyst-enzyme coupled artificial photosynthesis system for tailor-made chiral solar chemicals. The results will be published in the near future. Therefore, this paper not only shows a benchmark example of the graphene-based material used as a photocatalyst in general artificial photosynthesis, but also the benchmark example of the selective production system of solar chemicals/solar fuel directly from CO2.”
More information: Rajesh K. Yadav, et al. “A Photocatalyst-Enzyme Coupled Artificial Photosynthesis System for Solar Energy in Production of Formic Acid from CO2.” JACS. DOI: 10.1021/ja3009902
Journal information: Journal of the American Chemical Society
Copyright 2012 Phys.org
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of PhysOrg.com.