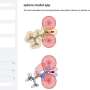
Revealing bacterial chainmail structure
An international team of scientists has uncovered the structure of the protective protein coat which surrounds many bacteria like a miniature suit of armour. Their research, which is published today in Nature, has far ranging consequences in helping us understand how some pathogenic bacteria infect humans and animals, and could help us develop new vaccines.
Until now, scientists have known very little about the structure and function of this coat, which scientists call S-layer, despite the fact that some bacteria invest as much as a third of their total protein production in building it.
The team of scientists from the UK, France and Belgium, were able to image the S-layer of a harmless soil bacterium called Geobacillus stearothermophilus down to the scale of a single atom. They revealed that the individual proteins of the protective layer hook together much like the chainmail of a medieval knight.
Dr Stefan Howorka, of UCL (University College London), led the work in the UK. He explains "These protein coats have remained quite mysterious to scientists even though they are found on a huge variety of bacteria. Using advanced imaging techniques, we have uncovered for the first time the structure of an S-layer in remarkable detail showing that the protein subunits are linked together in a manner resembling a chainmail. This remarkably optimized layer not only provides a tough but flexible coat of armour to protect the bacterium, but is also permeable allowing nutrients and other substances to diffuse in or out."
This chainmail coat supports the shape of bacteria and protects them from environmental hazards. The coat is also thought to be important in allowing many pathogenic bacteria to infect cells, helping germs to stick to and slide into human or animal cells where they can wreak havoc. Other pathogens coat themselves with a protein lattice that makes them invisible to the "radar" of the immune system.
Dr Howorka continues "Now that we have worked out how to obtain the structure of the S-layer in one bacterium, we expect that the structure of the protein coats of other species will soon be revealed. Uncovering the bacterial armour of pathogens like the superbug Clostridium difficile or of Bacillus anthraci, the bacterium responsible for anthrax, is now a high priority for many scientists. This understanding provides a real opportunity to find chinks in the bacterial armour that would allow precise targeting of antibiotics or vaccines against these challenging pathogens."
The remarkable structure of the S-layer coat also holds promise as a carrier for vaccines. By exploiting the ability of these coats to self-assemble from their individual building blocks it should be possible to construct hybrid vaccines that fuse harmless S-layers with bits of proteins from pathogenic bacteria.
Professor Douglas Kell, BBSRC Chief Executive said "This work is a great example of how important it is to study the secrets of how nature fits together at the most minute scale. By revealing how things look we can gain an insight into how they work. Understanding how nature works is going to be crucial in combating many of the great challenges facing society."
More information: dx.doi.org/10.1038/nature11155
Journal information: Nature