Researchers find simple and cheap way to mass-produce graphene nanosheets
Mixing a little dry ice and a simple industrial process cheaply mass-produces high-quality graphene nanosheets, researchers in South Korea and Case Western Reserve University report.
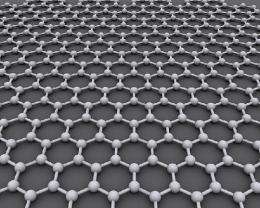
Graphene, which is made from graphite, the same stuff as "lead" in pencils, has been hailed as the most important synthetic material in a century. Sheets conduct electricity better than copper, heat better than any material known, are harder than diamonds yet stretch.
Scientists worldwide speculate graphene will revolutionize computing, electronics and medicine but the inability to mass-produce sheets has blocked widespread use.
A description of the new research will be published the week of March 26 in the online Early Edition of the Proceedings of the National Academy of Sciences.
Jong-Beom Baek, professor and director of the Interdisciplinary School of Green Energy/Advanced Materials & Devices, Ulsan National Institute of Science and Technology, Ulsan, South Korea, led the effort.
"We have developed a low-cost, easier way to mass produce better graphene sheets than the current, widely-used method of acid oxidation, which requires the tedious application of toxic chemicals," said Liming Dai, professor of macromolecular science and engineering at Case Western Reserve and a co-author of the paper.
Here's how:
Researchers placed graphite and frozen carbon dioxide in a ball miller, which is a canister filled with stainless steel balls. The canister was turned for two days and the mechanical force produced flakes of graphite with edges essentially opened up to chemical interaction by carboxylic acid formed during the milling.
The carboxylated edges make the graphite soluble in a class of solvents called protic solvents, which include water and methanol, and another class called polar aprotic solvents, which includes dimethyl sulfoxide.
Once dispersed in a solvent, the flakes separate into graphene naonsheets of five or fewer layers.
To test whether the material would work in direct formation of molded objects for electronic applications, samples were compressed into pellets. In a comparison, these pellets were 688 times better at conducting electricity than pellets yielded from the acid oxidation of graphite.
After heating the pellets at 900 degrees Celsius for two hours, the edges of the ball-mill–derived sheets were decarboxylated, that is, the edges of the nanosheets became linked with strong hydrogen bonding to neighboring sheets, remaining cohesive. The compressed acid-oxidation pellet shattered during heating.
To form large-area graphene nanosheet films, a solution of solvent and the edge-carboxylated graphene nanosheets was cast on silicon wafers 3.5 centimeters by 5 centimeters, and heated to 900 degrees Celsius. Again, the heat decarboxylated the edges, which then bonded with edges of neighboring pieces. The researchers say this process is limited only by the size of the wafer. The electrical conductivity of the resultant large-area films, even at a high optical transmittance, was still much higher than that of their counterparts from the acid oxidation.
By using ammonia or sulfur trioxide as substitutes for dry ice and by using different solvents, "you can customize the edges for different applications," Baek said. "You can customize for electronics, supercapacitors, metal-free catalysts to replace platinum in fuel cells. You can customize the edges to assemble in two-dimensional and three-dimensional structures."
Provided by Case Western Reserve University