Research provides octagonal window of opportunity for carbon capture
(PhysOrg.com) -- Filtering carbon dioxide, a greenhouse gas, from factory smokestacks is a necessary, but expensive part of many manufacturing processes. However, a collaborative research team from the National Institute of Standards and Technology and the University of Delaware has gathered new insight into the performance of a material called a zeolite that may stop carbon dioxide in its tracks far more efficiently than current scrubbers do.
Zeolites are highly porous rocks—think of a sponge made of stone—and while they occur in nature, they can be manufactured as well. Their toughness, high surface area (a gram of zeolite can have hundreds of square meters of surface in its myriad internal chambers) and ability to be reused hundreds of times makes them ideal candidates for filtering gas mixtures. If an unwanted molecule in the gas mixture is found to stick to a zeolite, passing the mixture through it can scrub the gas of many impurities, so zeolites are widely used in industrial chemistry as catalysts and filters.
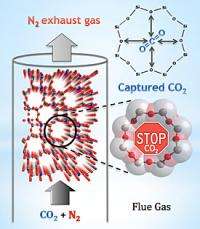
The team explored a zeolite created decades ago in an industrial lab and known by its technical name, SSZ-13. This zeolite, which has octagonal “windows” between its interior pore spaces, is special because it seems highly capable of filtering out carbon dioxide (CO2) from a gas mixture. “That makes SSZ-13 a promising candidate for scrubbing this greenhouse gas out of such things as factory smokestacks,” says Craig Brown, a researcher at the NIST Center for Neutron Research (NCNR). “So we explored, on an atomic level, how it does this so well.”
Using neutron diffraction, the team determined that SSZ-13’s eight-sided pore windows are particularly good at attracting the long, skinny carbon dioxide molecules and holding onto their “positively-charged” central carbon atoms, all the while allowing other molecules with different shapes and electronic properties to pass by unaffected. Like a stop sign, each pore halts one CO2 molecule—and each cubic centimeter of the zeolite has enough pores to stop 0.31 grams of CO2, a quantity that makes SSZ-13 highly competitive when compared to other adsorbent materials.
Brown says a zeolite like SSZ-13 probably will become a prime candidate for carbon scrubbing because it also could prove more economical than other scrubbers currently used in industry. SSZ-13’s ability to attract only CO2 could mean its use would reduce the energy demands of scrubbing, which can require up to 25 percent of the power generated in a coal or natural gas power plant.
“Many industrial zeolites attract water and carbon dioxide, which are both present in flue exhaust—meaning both molecules are, in a sense, competing for space inside the zeolite,” Brown explains. “We suspect that this novel CO2 adsorption mechanism means that water is no longer competing for the same site. A zeolite that adsorbs CO2 and little else could create significant cost savings, and that’s what this one appears to do.”
Brown says his team is still collecting data to confirm this theory, and that their future efforts will concentrate on exploring whether SSZ-13 is equally good at separating CO2 from methane—the primary component of natural gas. CO2 is also released in significant quantities during gas extraction, and the team is hopeful SSZ-13 can address this problem as well.
More information: M.R. Hudson, et al. Unconventional, highly selective CO2 adsorption in zeolite SSZ-13. Journal of the American Chemical Society Published on the Web Jan. 10, 2012. DOI: 10.1021/ja210580b
Journal information: Journal of the American Chemical Society
Provided by National Institute of Standards and Technology