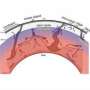
How heavy and light isotopes separate in magma
In the crash-car derby between heavy and light isotopes vying for the coolest spots as magma turns to solid rock, weightier isotopes have an edge, research led by Case Western Reserve University shows.
This tiny detail may offer clues to how igneous rocks form.
As molten rock cools along a gradient, atoms want to move towards the cool end. This happens because hotter atoms move faster than cooler atoms and, therefore, hotter atoms move to the cool region faster than the cooler atoms move to the hot region.
Although all isotopes of the same element want to move towards the cool end, the big boys have more mass and, therefore, momentum, enabling them to keep moving on when they collide along the way.
"It's as if you have a crowded, sealed room of sumo wrestlers and geologists and a fire breaks out at one side of the room," said Daniel Lacks, chemical engineering professor and lead author of the paper. "All will try to move to the cooler side of the room, but the sumo wrestlers are able to push their way through and take up space on the cool side, leaving the geologists on the hot side of the room."
Lacks worked with former postdoctoral researcher Gaurav Goel and geology professor James A. Van Orman at Case Western Reserve; Charles J. Bopp IV and Craig C. Lundstrum, of University of Illinois, Urbana; and Charles E. Lesher of the University of California at Davis. They described their theory and confirming mathematics, computer modeling, and experiments in the current issue of Physical Review Letters.
Lacks, Van Orman and Lesher also published a short piece in the current issue of Nature, showing how their findings overturn an explanation based on quantum mechanics, published in that journal last year.
"The theoretical understanding of thermal isotope separation in gases was developed almost exactly 100 years ago by David Enskog, but there is as yet not a similar full understanding of this process in liquids," said Frank Richter, who is the Sewell Avery Distinguished Professor at the University of Chicago and a member of the National Academy of Sciences. He was not involved in the research. "This work by Lacks et al. is an important step towards remedying this situation."
This separation among isotopes of the same element is called fractionation.
Scientists have been able to see fractionation of heavy elements in igneous rocks only since the 1990s, Van Orman said. More sensitive mass spectrometers showed that instead of a homogenous distribution, the concentration ratio of heavy isotopes to light isotopes in some igneous rocks was up to 0.1 percent higher than in other rocks.
One way of producing this fractionation is by temperature.
To understand how this happens, the team of researchers created a series of samples made of molten magnesium silicate infused with elements of different mass, from oxygen on up to heavy uranium.
The samples, called silicate melts, were heated at one end in a standard lab furnace, creating temperature gradients in each. The melts were then allowed to cool and solidify.
The scientists then sliced the samples along gradient lines and dissolved the slices in acid. Analysis showed that no matter the element, the heavier isotopes slightly outnumbered the lighter at the cool end of the gradient.
Computer simulations of the atoms, using classical mechanics, agreed with the experimental results.
"The process depends on temperature differences and can be seen whether the temperature change across the sample is rapid or gradual," Lacks said.
Thermal diffusion through gases was one of the first methods used to separate isotopes, during the Manhattan Project. It turns out that isotope fractionation through silicate liquids is even more efficient than through gases.
"Fractionation can occur inside the Earth wherever a sustained temperature gradient exists," Van Orman said. "One place this might happen is at the margin of a magma chamber, where hot magma rests against cold rock. Another is nearly 1,800 miles inside the Earth, at the boundary of the liquid core and the silicate mantle."
The researchers are now adding pressure to the variables as they investigate further. This work was done at atmospheric pressure but where the Earth's core and mantle meet, the pressure is nearly 1.4 million atmospheres.
Lacks and Van Orman are unsure whether high pressure will result in greater or lesser fractionation. They can see arguments in favor of either.
Journal information: Physical Review Letters
Provided by Case Western Reserve University