Mock atoms prove attractive: Researchers added first pseudo atoms to electronegativity scale

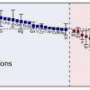
(PhysOrg.com) -- When studying an atom's ability to attract nearby electrons, scientists rely on electronegativity scales, which describe each atom's ability to pull in these negatively charged particles. But what about pseudo-atoms, a.k.a. those molecular fragments that don't change much in various environments and are considered to behave like atoms? Scientists at Pacific Northwest National Laboratory and Heriot-Watt University in Scotland put the tetrahedral ammonium radical, made from a nitrogen atom and four hydrogen atoms, on the electronegativity scale. It has the same attractive force as potassium and has the same size as an atom of rubidium.
Electron attraction and repulsion determine how atoms and pseudo-atoms behave in different environments. This study provides scientists with the information they need to better predict, manipulate and control those behaviors, whether in batteries for solar farms or catalysts for bio-fuel production.
This study began when Alexander Whiteside, a student at Heriot-Watt University, came to PNNL as part of the 2008 Summer Research Institute for Interfacial and Condensed Phase Physics. During that summer visit, Whiteside, his advisor Maciej Gutowski, and Laboratory Fellow Sotiris Xantheas started to determine the electronegativity of the ammonium radical.
The team calculated the electronic structure of the neutral ammonium molecule, NH4, and its positive and negative ions. Next, they computed the properties of ammonium complexes, specifically combining ammonium with astatine or selected borohydrides; the latter are promising materials for hydrogen storage.

"These results clarified the properties of NH4 and placed it in the proper scale compared to the alkali metals," said Xantheas. "In comparison with alkali atoms, ammonium's electronegativity punches above its effective cationic radius. Nobody had really put it into the scale yet. This study did just that."
The results graced the cover of Chemistry: A European Journal and were highlighted in the Royal Society of Chemistry's Chemistry World.
"Past generations of physical and theoretical chemists were intrigued by the properties ammonium. Alex was standing on the shoulders of giants—Berzelius, Pauling, Mulliken, Herzberg—whose pictures are highlighted in the cover of the journal, while Sotiris and I helped him to keep his balance," joked Gutowski.
"This work opens up the opportunity to developing a comprehensive view on other pseudo-alkali metal species, pseudo-halogens and other pseudo-atoms" says Alexander Boldyrev of Utah State University.
Indeed, the team is expanding their study to the properties of the nearly ubiquitous hydronium and methyl groups, which contain a single oxygen or carbon atom and three hydrogen atoms. In addition, they are examining cyanide, a carbon and nitrogen atom combination that could provide new insights into that pseudo-atom's behavior.
More information: Whiteside A, SS Xantheas, and M Gutowski. 2011. "Is Electronegativity a Useful Descriptor for the Pseudo-Alkali Metal NH4?" Chemistry: A European Journal 17:13197-13205. DOI:10.1002/chem.201101949
Provided by Pacific Northwest National Laboratory