January 18, 2012 feature
Bubble-propelled microrockets could operate in the human stomach
(PhysOrg.com) -- Recently, researchers have been designing a wide variety of self-propelled micromotors, many of which operate using an oxygen-bubble propulsion mechanism that requires a high concentration of hydrogen peroxide fuel. Since hydrogen peroxide is hazardous at high concentrations, this requirement has hindered practical applications, especially biomedical uses. Now in a new study, scientists have designed and built a new type of micromotor that propels itself through acidic environments with hydrogen bubbles, and requires no additional fuels. At extremely low pH levels, the micromotors can travel at speeds of up to 100 body lengths per second, prompting the scientists to call them “microrockets.”
The researchers, Wei Gao, Aysegul Uygun, and Joseph Wang from the University of California, San Diego, have published their study on the hydrogen-bubble-propelled microrockets in a recent issue of the Journal of the American Chemical Society.
“This is the first reported example of chemically-powered microrockets that can be self-propelled without an external fuel (such as the common hydrogen peroxide),” Wang told PhysOrg.com. “Such acid-powered microrockets could greatly expand the scope of applications of nano-/microscale motors toward new extreme environments (e.g., the human stomach or silicon wet-etching baths) and could thus lead to diverse new biomedical or industrial applications ranging from targeted drug delivery or nanoimaging to the monitoring of industrial processes.”
The microrockets are in the shape of tiny tubes, measuring about 10 micrometers long with diameters varying from 2 to 5 micrometers. The researchers fabricated the tubes out of the common polymer polyaniline (PANI) in templates, and then electrodeposited a thin layer of zinc on the inner surface. When the microrockets are immersed in any highly acidic solution, the zinc loses electrons and – due to having a more negative redox potential than hydrogen – promotes the production of hydrogen bubbles. The researchers experimented with using other metals, such as iron and lead, but they did not produce as many bubbles as zinc.
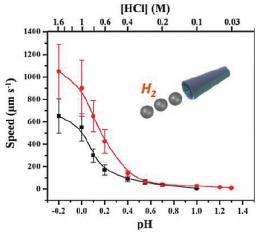
Tests showed that the microrockets’ speed increases as the pH of the solution decreases. The fastest speed of 1,050 micrometers per second (equivalent to about 100 body lengths per second) was achieved by a 5-micrometer-diameter microrocket at a pH of -0.2. The speed decreased to about 10 micrometers per second at a pH of 1.3. Although the microckets have a limited pH range, the researchers noted that they could be useful in the stomach, which has a pH range of 0.8-2.0, as well as in some types of human serum.
Tests also showed that the lifetime of the microrockets can vary from 10 seconds to 2 minutes, depending on the rate of zinc dissolution. The more zinc the rocket has, and the higher the pH of the solution, the longer the microrocket’s lifetime.
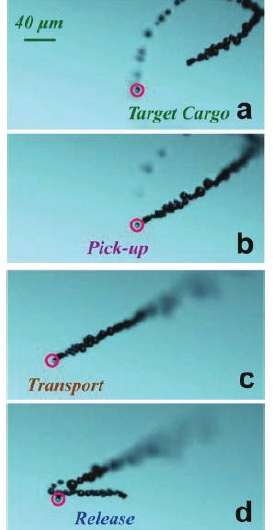
While the microrockets can move autonomously in this way, the researchers also showed that it’s possible to control their direction and even to make them pick up and release cargo. The scientists did this by depositing a magnetic layer on the microcket’s outer surface, and then magnetically guiding the device in the preferred direction. They showed that a microcket could magnetically capture a polystyrene cargo, transport it on a predetermined path, and then release it by rapidly changing the magnetic field direction.
The scientists predict that this capability could prove especially useful for a variety of biomedical applications as well as monitoring industrial processes such as semiconductor processing. In addition, because the microrocket’s speed is directly related to the solution’s pH, the devices could be used for sensitive pH sensing, such as detecting changes in stomach acidity. With its biggest advantage of being fueled by its acidic environment, without the need for additional fuel, the microrockets could further expand the scope of micromotor applications in many directions.
“With further improvements and optimization, we hope to improve and expand the working environments to milder conditions and extend the lifetime of such microrockets to longer periods,” Wang said. “We are also exploring new materials to broaden the scope of our microengines towards new environments.”
More information: Wei Gao, et al. “Hydrogen-Bubble-Propelled Zinc-Based Microrockets in Strongly Acidic Media.” Journal of the American Chemical Society. DOI: 10.1021/ja210874s
Journal information: Journal of the American Chemical Society
Copyright 2012 PhysOrg.com.
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of PhysOrg.com.