This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
reputable news agency
proofread
Trio wins Nobel Prize in chemistry for work on quantum dots, used in electronics and medical imaging

Three scientists won the Nobel Prize in chemistry Wednesday for their work on quantum dots—tiny particles just a few nanometers in diameter that can release very bright colored light and whose applications in everyday life include electronics and medical imaging.
Moungi Bawendi of MIT, Louis Brus of Columbia University, and Alexei Ekimov of Nanocrystals Technology Inc., were honored for their work with the tiny particles that "have unique properties and now spread their light from television screens and LED lamps," according to the Royal Swedish Academy of Sciences, which announced the award in Stockholm.
The suspense surrounding the academy's decision took an unusual turn when Swedish media reported the winners several hours before the prize was announced. The advance notice apparently came from a news release sent out early by mistake.

WHAT DISCOVERY WON THE NOBEL PRIZE IN CHEMISTRY?
Quantum dots are tiny inorganic particles that glow a range of colors from red to blue when exposed to light. The color they emit depends upon the size of the particle.
Scientists can engineer the dots from materials that include gold to graphene to cadmium, and create their color by controlling their size. The tiniest particles, in which electrons are most tightly confined, emit blue light. Slightly larger particles, in which electrons bounce around a longer wavelength, emit red light.

Chemists sometimes compare the size of the particle itself to a confining box.
The underlying "particle in a box" theory of quantum mechanics was first described nearly a century ago. But it wasn't until several decades later that scientists could manufacture quantum dots in a lab.
In the 1980s, Ekimov, 78, and Brus, 80, honed the theory and developed early laboratory techniques for creating particles that emit varying colors by adjusting sizes. In 1993, Bawendi, 62, developed new chemical methods for producing the particles quickly and uniformly—which soon enabled a variety of scalable commercial applications, including in electronics displays.
Judy Giordan, president of the American Chemical Society, said she was thrilled at this year's winners.
"What we care about a lot in chemistry is being able to make and tailor novel structures and architectures to solve problems that help people and the planet," Giordan said.

Rigoberto Advincula, a materials chemist at Oak Ridge National Laboratory in Tennessee, said the work helped bridge the fields of physics and chemistry, adding: "This technology is very easy to reproduce—that's why it became so popular and so widespread."
Today quantum dots are commonly used in electronics displays and biomedical imaging. The florescent quality of the particles allows researchers to track how drugs are delivered within the human body, as well as to study the precise location and growth of a tumor, for example.
WERE THE WINNERS ANNOUNCED PREMATURELY?
Swedish media reported hours before Wednesday's announcement that the Royal Swedish Academy of Sciences had sent out a news release that identified Bawendi, Brus and Ekimov as the latest Nobel laureates.
Public broadcaster SVT said the release said they were receiving the prize for the "discovery and synthesis of quantum dots."

After officially announcing the three winners, Secretary-General Hans Ellegren said the Swedish academy would investigate how the information got out in advance.
"There was a press release sent out for still unknown reasons. We have been very active this morning to find out exactly what happened," he said. "This is very unfortunate and we deeply regret what happened."
The academy, which awards the physics, chemistry and economics prizes, asks for nominations a year in advance from thousands of university professors and other scholars around the world.
A committee for each prize then discusses candidates in a series of meetings before presenting one or more proposals to the full academy for a vote. The deliberations, including the names of nominees other than the winners, are kept confidential for 50 years.

HOW DID THE WINNERS REACT?
Bawendi told the news conference he was "very surprised, sleepy, shocked, unexpected and very honored."
Asked about the leak, he said he didn't know he'd been made a Nobel laureate until he was called by the academy.
Bawendi said he was not thinking about the possible applications of his work when he started researching quantum dots.
"The motivation really is the basic science. A basic understanding, the curiosity of how does the world work? And that's what drives scientists and academic scientists to do what they do," he said.
Brus, a professor emeritus at Columbia, said he didn't pick up the phone when the early morning call came from the Swedish academy to notify him.
"It was ringing during the night, but I didn't answer it because I'm trying to get some sleep, basically," he told The Associated Press. He finally saw the news online when he got up around 6 a.m.

"I certainly was not expecting this," Brus said.
Brus said he was glad to see recognition for the area of chemistry he practices. The practical applications of quantum dots, like creating the colors in flatscreen TVs, are something he was hoping for when he started the work decades ago, he said.
"Basic research is extremely hard to predict exactly how it's going to work out," Brus said. "It's more for the knowledge base than it is for the actual materials. But in this case, it's both."
Ekimov is the former chief scientist at Nanocrystals Technology, a company based in New York where he started working in 1999. The Swedish academy credited him with demonstrating in the early 1980s that the size of copper chloride nanoparticles affected the colors in glass.
"It was really nice that we got it after 40 years of work," Ekimov told the AP. His first publication on the topic was in 1981.
-

Nobel Committee for Chemistry Heiner Linke, right, presents the work of the winners of the 2023 Nobel Prize in Chemistry during a press conference at the Royal Academy of Sciences, in Stockholm, Wednesday, Oct. 4, 2023. The Nobel Prize in physics has been awarded to scientists Moungi Bawendi, Louis Brus and Alexi Ekimov for discovery and synthesis of quantum dots. Credit: Claudio Bresciani/TT News Agency via AP -

Professor Emeritus Louis Brus rests at his home, after wining the Nobel Prize in Chemistry, in Hastings-on-Hudson, NY, Wednesday, Oct. 4, 2023. Credit: AP Photo/Eduardo Munoz Alvarez -

Winner of the 2023 Nobel Prize in Chemistry Massachusetts Institute of Technology scientist Moungi Bawendi speaks on a phone at his home, in Cambridge, Mass, Wednesday, Oct. 4, 2023. Bawendi, of MIT; Louis Brus, of Columbia University; and Alexei Ekimov, of Nanocrystals Technology Inc., were honored for their work with the particles that "have unique properties and now spread their light from television screens and LED lamps," according to the Royal Swedish Academy of Sciences, which announced the award in Stockholm. Credit: AP Photo/Steven Senne -
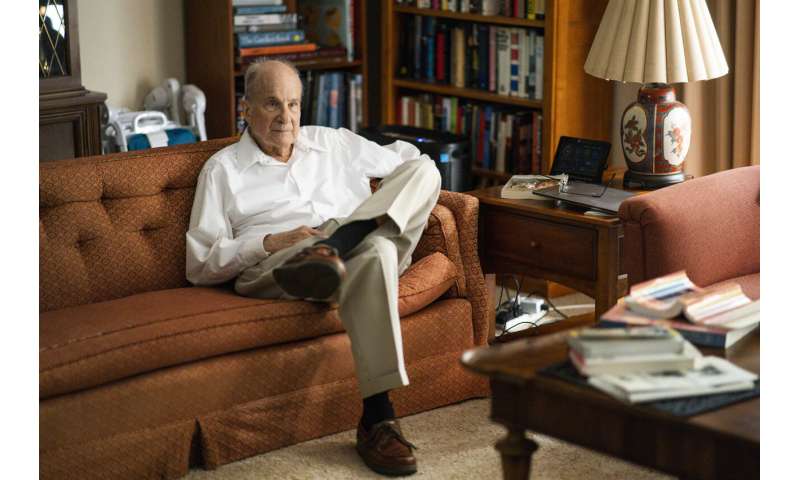
Professor Emeritus Louis Brus rests at his home, after wining the Nobel Prize in Chemistry, in Hastings-on-Hudson, NY, Wednesday, Oct. 4, 2023. Credit: AP Photo/Eduardo Munoz Alvarez -

Professor Emeritus Louis Brus takes a look at his books at his home, after wining the Nobel Prize in Chemistry, in Hastings-on-Hudson, NY, Wednesday, Oct. 4, 2023. Credit: AP Photo/Eduardo Munoz Alvarez -

Professor Emeritus Louis Brus rests at his home, after wining the Nobel Prize in Chemistry, in Hastings-on-Hudson, NY, Wednesday, Oct. 4, 2023. Credit: AP Photo/Eduardo Munoz Alvarez
On Tuesday, the physics prize went to French-Swedish physicist Anne L'Huillier, French scientist Pierre Agostini and Hungarian-born Ferenc Krausz for producing the first split-second glimpse into the super-fast world of spinning electrons.
On Monday, Hungarian-American Katalin Karikó and American Drew Weissman won the Nobel Prize in medicine for discoveries that enabled the creation of mRNA vaccines against COVID-19.
The prizes in literature, peace and economics follow, with one announcement every weekday until Monday.
The Nobel Prizes carry a cash award of 11 million Swedish kronor ($1 million) from a bequest left by the prize's creator, Swedish inventor Alfred Nobel.
Nobel Committee announcement:
The Royal Swedish Academy of Sciences has decided to award the Nobel Prize in Chemistry 2023 to
Moungi G. Bawendi
Massachusetts Institute of Technology (MIT), Cambridge, MA, U.S.
Louis E. Brus
Columbia University, New York, NY, U.S.
Alexei I. Ekimov
Nanocrystals Technology Inc., New York, NY, U.S.
"for the discovery and synthesis of quantum dots"
They planted an important seed for nanotechnology
The Nobel Prize in Chemistry 2023 rewards the discovery and development of quantum dots, nanoparticles so tiny that their size determines their properties. These smallest components of nanotechnology now spread their light from televisions and LED lamps, and can also guide surgeons when they remove tumour tissue, among many other things.
Everyone who studies chemistry learns that an element's properties are governed by how many electrons it has. However, when matter shrinks to nano-dimensions quantum phenomena arise; these are governed by the size of the matter. The Nobel Laureates in Chemistry 2023 have succeeded in producing particles so small that their properties are determined by quantum phenomena. The particles, which are called quantum dots, are now of great importance in nanotechnology.
"Quantum dots have many fascinating and unusual properties. Importantly, they have different colours depending on their size," says Johan Åqvist, Chair of the Nobel Committee for Chemistry.
Physicists had long known that in theory size-dependent quantum effects could arise in nanoparticles, but at that time it was almost impossible to sculpt in nanodimensions. Therefore, few people believed that this knowledge would be put to practical use.
However, in the early 1980s, Alexei Ekimov succeeded in creating size-dependent quantum effects in coloured glass. The colour came from nanoparticles of copper chloride and Ekimov demonstrated that the particle size affected the colour of the glass via quantum effects.
A few years later, Louis Brus was the first scientist in the world to prove size-dependent quantum effects in particles floating freely in a fluid.
In 1993, Moungi Bawendi revolutionised the chemical production of quantum dots, resulting in almost perfect particles. This high quality was necessary for them to be utilised in applications.
Quantum dots now illuminate computer monitors and television screens based on QLED technology. They also add nuance to the light of some LED lamps, and biochemists and doctors use them to map biological tissue.
Quantum dots are thus bringing the greatest benefit to humankind. Researchers believe that in the future they could contribute to flexible electronics, tiny sensors, thinner solar cells and encrypted quantum communication—so we have just started exploring the potential of these tiny particles.
They added colour to nanotechnology
Moungi G. Bawendi, Louis E. Brus and Alexei I. Ekimov are awarded the Nobel Prize in Chemistry 2023 for the discovery and development of quantum dots. These tiny particles have unique properties and now spread their light from television screens and LED lamps. They catalyse chemical reactions and their clear light can illuminate tumour tissue for a surgeon.
"Toto, I've a feeling we're not in Kansas anymore," is a classic quote from the flm The Wizard of Oz.
Twelve-year-old Dorothy faints onto her bed when her house is swept away by a powerful tornado, but when the house lands again and she steps outside the door, her dog Toto in her arms, everything has changed. Suddenly she is in a magical, technicolour world.
If an enchanted tornado were to sweep into our lives and shrink everything to nano dimensions, we would almost certainly be as astonished as Dorothy in the land of Oz. Our surroundings would be dazzlingly colourful and everything would change. Our gold earrings would suddenly glimmer in blue, while the gold ring on our fnger would shine a ruby red. If we tried to fry something on the gas hob, the frying pan might melt. And our white walls—whose paint contains titanium dioxide—would start generating lots of reactive oxygen species.
Size matters on the nanoscale
In the nanoworld, things really do behave diferently. Once the size of matter starts to be measured in millionths of a millimetre, strange phenomena start to occur—quantum efects—that challenge our intuition. The 2023 Nobel Laureates in Chemistry have all been pioneers in the exploration of the nanoworld. In the early 1980s, Louis Brus and Alexei Ekimov succeeded in creating—independently of each other—quantum dots, which are nanoparticles so tiny that quantum efects determine their characteristics. In 1993, Moungi Bawendi revolutionised the methods for manufacturing quantum dots, making their quality extremely high—a vital prerequisite for their use in today's nanotechnology.
Thanks to the work of the laureates, humanity is now able to utilise some of the peculiar properties of the nanoworld. Quantum dots are now found in commercial products and used across many scientifc disciplines, from physics and chemistry to medicine—but we are getting ahead of ourselves. Let's uncover the background to the Nobel Prize in Chemistry 2023.
For decades, quantum phenomena in the nanoworld were just a prediction
When Alexei Ekimov and Louis Brus produced the frst quantum dots, scientists already knew that they could—in theory—have unusual characteristics. In 1937, the physicist Herbert Fröhlich had already predicted that nanoparticles would not behave like other particles. He explored the theoretical consequences of the famous Schrödinger equation, which shows that when particles become extremely small there is less space for the material's electrons. In turn, the electrons—which are both waves and particles—are squeezed together. Fröhlich realised that this would result in drastic changes to the material's properties.
Researchers were fascinated by this insight and, using mathematical tools, they succeeded in predicting numerous size-dependent quantum efects. They also worked to try to demonstrate them in reality, but this was easier said than done because they needed to sculpt a structure that was about a million times smaller than a pinhead.
Few people thought quantum effects could be utilised
Still, in the 1970s, researchers did succeed in making such a nanostructure. Using a type of molecular beam, they created a nano-thin layer of coating material on top of a bulk material. Once the assembly was complete, they were able to show that the coating's optical properties varied depending on how thin it was, an observation that matched the predictions of quantum mechanics.
This was a major breakthrough, but the experiment required very advanced technology. Researchers needed both an ultra-high vacuum and temperatures close to absolute zero, so few people expected that quantum mechanical phenomena would be put to practical use. However, now and again science ofers up the unexpected and, this time, the turning point was due to studies of an ancient invention: coloured glass.
A single substance can give glass different colours
The oldest archaeological fnds of coloured glass are from several thousand years ago. Glassmakers have tested their way to an understanding of how glass can be produced in all the colours of the rainbow.
They added substances such as silver, gold and cadmium and then played with diferent temperatures to produce beautiful shades of glass.
In the nineteenth and twentieth centuries, when physicists started to investigate the optical properties of light, the glassmakers' knowledge was put to use. Physicists could use coloured glass to flter out selected wavelengths of light. To optimise their experiments, they started to produce glass themselves, which led to important insights. One thing they learned was that a single substance could result in completely diferently coloured glass. For example, a mixture of cadmium selenide and cadmium sulphide could make glass turn either yellow or red—which one it became depended on how much the molten glass was heated and how it was cooled. Eventually, they were also able to show that the colours came from particles forming inside the glass and that the colour depended on the particles' size.
This was more or less the state of the knowledge at the end of the 1970s, when one of this year's laureates, Alexei Ekimov, a recent doctoral graduate, started working at the S. I. Vavilov State Optical Institute in what was then the Soviet Union.
Alexei Ekimov maps the mysteries of coloured glass
The fact that a single substance could result in diferent coloured glass interested Alexei Ekimov, because it is actually illogical. If you paint a picture in cadmium red, it will always be cadmium red, unless you mix in other pigments. So how could a single substance give glass of diferent colours?
During his doctoral degree, Ekimov studied semiconductors—important components in microelectronics.
In this feld, optical methods are used as diagnostic tools for assessing the quality of semiconducting material. Researchers shine light on the material and measure the absorbance. This reveals what substances the material is made from and how well-ordered the crystal structure is.
Ekimov was familiar with these methods, so he began using them to examine coloured glass. After some initial experiments, he decided to systematically produce glass that was tinted with copper chloride. He heated the molten glass to a range of temperatures between 500°C and 700°C, varying the heating time from 1 hour to 96 hours. Once the glass had cooled and hardened, he X-rayed it.
The scattered rays showed that tiny crystals of copper chloride had formed inside the glass and the manufacturing process afected the size of these particles. In some of the glass samples they were only about two nanometres, in others they were up to 30 nanometres.
Interestingly, it turned out that the glass' light absorption was afected by the size of the particles.
The biggest particles absorbed the light in the same way that copper chloride normally does, but the smaller the particles, the bluer the light that they absorbed. As a physicist, Ekimov was well acquainted with the laws of quantum mechanics and quickly realised that he had observed a sizedependent quantum efect (fgure 3).
This was the frst time someone had succeeded in deliberately producing quantum dots—nanoparticles that cause size-dependent quantum efects. In 1981, Ekimov published his discovery in a Soviet scientifc journal, but this was difcult for researchers on the other side of the Iron Curtain to access. Therefore, this year's next Nobel Prize Laureate in Chemistry—Louis Brus—was unaware of Alexei Ekimov's discovery when, in 1983, he was the frst researcher in the world to discover size-dependent quantum efects in particles foating freely in a solution.
Brus shows that the strange properties of particles are quantum effects
Louis Brus was working at Bell Laboratories in the US, with the long-term aim of making chemical reactions happen using solar energy. To achieve this, he was using particles of cadmium sulphide, which can capture light and then utilise its energy to drive reactions. The particles were in a solution and Brus made them very small, because this gave him a larger area on which the chemical reactions could take place; the more a material is chopped up, the greater the surface area it will expose to its surroundings.
During his work with these tiny particles, Brus noticed something strange—their optical properties changed after he had left them on the lab bench for a while. He guessed that this could be because the particles had grown, so to confrm his suspicions he produced cadmium sulphide particles that were just about 4.5 nanometres in diameter. Brus then compared the optical properties of these newly made particles with those of the larger particles, which had a diameter of about 12.5 nanometres. The larger particles absorbed light at the same wavelengths as cadmium sulphide generally does, but the smaller particles had an absorption that shifted towards blue.
Just like Ekimov, Brus understood that he had observed a size-dependent quantum efect. He published his discovery in 1983 and then started investigating particles made from a range of other substances. The pattern was the same—the smaller the particles, the bluer the light they absorbed.
The periodic table gained a third dimension
This is where you may be tempted to ask "Why does it matter if a substance's absorbance is slightly more towards blue? Why is that so amazing?"
Well, the optical changes revealed that the substance's characteristics had completely changed. A substance's optical properties are governed by its electrons. The same electrons also govern the substance's other properties, such as its ability to catalyse chemical reactions or conduct electricity.
So when researchers detected the changed absorption they understood that, in principle, they were looking at an entirely new material.
If you want to understand the magnitude of this discovery, you can imagine that the periodic table suddenly gained a third dimension. An element's properties are not only afected by the number of electron shells and how many electrons there are it the outer shell but, at the nano level, size also matters. A chemist who wanted to develop a new material thus had another factor to play with—of course this tickled researchers' imaginations!
There was just one problem. The methods Brus had used to fabricate nonparticles generally resulted in unpredictable quality. Quantum dots are tiny crystals (fgure 2) and the ones that could be produced at that time often contained defects. They were also of varying sizes. It was possible to control how the crystals were formed so the particles had a given average size, but if researchers wanted all the particles in a solution to be about the same size they had to sort them after they were made. This was a difcult process that hindered development.
Moungi Bawendi revolutionises the production of quantum dots
This was a problem that this year's third Nobel Prize Laureate in Chemistry decided to solve.
Moungi Bawendi started his postdoctoral training at Louis Brus' laboratory in 1988, where intensive work was underway to improve the methods used to produce quantum dots. Using a range of solvents, temperatures and techniques, they experimented with a variety of substances to try and form well-organised nanocrystals. And the crystals were getting better, but were still not good enough.
However, Bawendi did not give up. When he started working as a research leader at the Massachusetts Institute of Technology, MIT, he continued his eforts to produce higher quality nanoparticles. The major breakthrough came in 1993, when the research group injected the substances that would form nanocrystals into a heated and carefully chosen solvent. They injected as much of the substances as was necessary to precisely saturate the solution, which led to tiny crystal embryos beginning to form simultaneously.
Then, by dynamically varying the temperature of the solution, Moungi Bawendi and his research group succeeded in growing nanocrystals of a specifc size. During this phase, the solvent helped give the crystals a smooth and even surface.
The nanocrystals that Bawendi produced were almost perfect, giving rise to distinct quantum efects.
Because the production method was easy to use, it was revolutionary—more and more chemists started working with nanotechnology and began to investigate the unique properties of quantum dots.
The luminous properties of quantum dots fnd commercial uses
Thirty years later, quantum dots are now an important part of nanotechnology's toolbox and are found in commercial products. Researchers have primarily utilised quantum dots to create coloured light. If quantum dots are illuminated with blue light, they absorb the light and emit a diferent colour. Modifying the size of the particles makes it possible to determine exactly what colour they should glow.
The luminous properties of quantum dots are utilised in computer and television screens based on QLED technology, where the Q stands for quantum dot. In these screens, blue light is generated using the energy-efcient diodes that were recognised with the Nobel Prize in Physics 2014. Quantum dots are used to change the colour of some of the blue light, transforming it into red or green. This makes it possible to produce the three primary colours of light needed in a television screen.
Similarly, quantum dots are used in some LED lamps to adjust the cold light of the diodes. The light can then become as energising as daylight or as calming as the warm glow from a dimmed bulb.
The light from quantum dots can also be used in biochemistry and medicine. Biochemists attach quantum dots to biomolecules to map cells and organs. Doctors have begun investigating the potential use of quantum dots to track tumour tissue in the body. Chemists instead use the catalytic properties of quantum dots to drive chemical reactions.
Quantum dots are thus bringing the greatest beneft to humankind, and we have just begun to explore their potential. Researchers believe that in the future quantum dots can contribute to fexible electronics, miniscule sensors, slimmer solar cells and perhaps encrypted quantum communication. One thing is certain—there is a lot left to learn about amazing quantum phenomena. So if there's a 12-year-old
Dorothy out there looking for adventure, the nanoworld has a great deal to ofer.
More information: Advanced information: www.nobelprize.org/uploads/202 … emistryprize2023.pdf
© 2023 The Associated Press. All rights reserved. This material may not be published, broadcast, rewritten or redistributed without permission.





















