Iodate refuses to intimidate
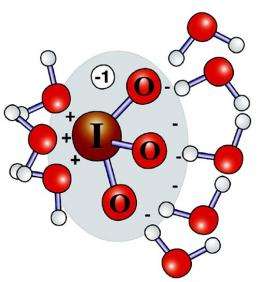
Like a bull in a china shop, a massive, iodine-based ion called iodate should disrupt the surrounding water molecules until it is forcibly expelled. However, it doesn't. This disconnect between the molecule's attributes and its behavior has puzzled scientists for some time. Scientists at Pacific Northwest and Argonne National Laboratories discovered the secret. While iodate is huge and surrounded by negative oxygen atoms, its central iodine atom takes on a positive character, or becomes a cation. This cationic region draws in water's oxygen atoms and allows the ion to reside peacefully in the liquid.
Whether they are creating a catalyst for petroleum-free fuel or designing better drug therapies, scientists need to control ions' actions in water. To control the ions, they must accurately characterize their behavior. This study answers a fundamental question about the behavior of large, negatively charged ions with multiple atoms, called polyoxyanions.
"To our knowledge, this is the first time anyone has microscopic insight into the solvation of a polyoxyanion," said Dr. Chris Mundy, a physical chemist at PNNL who co-authored the study.
When examining the behavior of large ions in water, the conventional wisdom is that large anions, negatively charged ions, such as iodate (IO3-) should disrupt the ordered structure of water. For example, iodide (I-) creates cavities within the water and is eventually expelled to the surface.
However, studies assigned iodate as strongly hydrating, meaning it resided in the liquid, not at the surface.
"The puzzle was why iodate did not behave like its slightly smaller cousin, iodide," said Mundy.
The research team took on that puzzle by integrating computer simulations and experimental studies with iodate. The simulations determined that the central iodine atom is positively charged, even though the ion has a negative charge. The simulations were density-functional theory-based molecular dynamics simulations run on a powerful computing system known as NWIce at EMSL.
The iodine's cationic behavior strongly attracts the negative oxygen atoms on three nearby water molecules. By tightly surrounding itself with water, the iodate is able to blend into the water.
"I would usually expect every water molecule to point a hydrogen at the anion," said Dr. Marcel Baer, a Linus Pauling Postdoctoral Fellow at PNNL who worked on the study. "The structure was surprising to me . . .very unexpected."
The simulations were confirmed by X-ray absorption fine structure spectroscopy studies. Using the light source at the Advanced Photon Source, the team was able to examine the structure of the ion in water.
"This one is not the same as the pure hydration that would normally be seen with a cation. It has its own structure," said Dr. Van-Thai Pham, a postdoctoral researcher at PNNL who worked on the spectroscopy investigations at the light source.
The research team's results appear in The Journal of Physical Chemistry Letters.
The researchers are further delving into the mysteries of iodate. The team is looking into the water surface to see if iodate is absorbed or the water shell that forms rejects the ion at the surface. In addition, team members are studying how other ions behave in water. They are focusing on ions based on positively charged cesium, bromide, and rubidium. Understanding these ions and others has implications from alternative energy sources to new drug therapies.
More information: MD Baer, et al. 2011. "Is Iodate a Strongly Hydrated Cation?" The Journal of Physical Chemistry Letters 2, 2650-2654. DOI: 10.1021/jz2011435.
Provided by Pacific Northwest National Laboratory