Detecting an unexpected delay at ultrafast speed
Molecules that suddenly transform into new structures when stimulated by photons or electrons play key roles in many chemical and biological processes. Recently, chemists have discovered that adding transition metals such as copper to photo-responsive organic ligands produces materials with high solar conversion efficiencies, owing to the metal’s ready supply of light-activated electrons. But despite the interest in these substances for opto-electronic devices, their inner workings remain mostly inscrutable because the charge-transfer dynamics happen too quickly for detection by typical instruments.
Tahei Tahara and colleagues from the RIKEN Advanced Science Institute, Wako, have spearheaded development of ultrafast laser spectroscopy that can capture these high-speed reactions by taking ‘snapshots’ of photochemical transformations with quadrillionths-of-a-second (10-15 s) accuracy. Now, an unprecedented finding by the research team—a picosecond (10-12 s) time delay during a theoretically instantaneous distortion—is set to overturn current thinking about light-driven rearrangements in transition metal complexes1.
Copper dimethylphenanthroline (Fig. 1) is a compound containing two propeller-shaped wings, made out of thin aromatic sheets. Chemists regularly use it to explore photo-induced structural changes. In its unexcited state, the complex’s wings are oriented perpendicular to each other. But when illuminated at a specific wavelength, the copper ion absorbs a photon and transfers an electron to the sheets—an action that flattens the structure by disrupting critical copper—phenanthroline bonds.
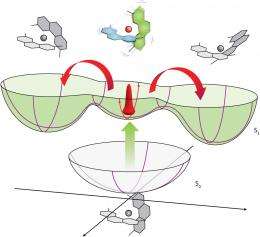
The exact flattening mechanism, however, has been controversial because copper electrons can be photo-excited in two different ways: through an easily accessible high-energy state called S2, or a harder-to-spot, low-energy transition called the S1 state. Tahara and colleagues tracked the extremely fast relaxation process from both states and found that S1 electrons provoked the flattening. This finding will allow researchers to eventually squeeze as much efficiency as possible from these devices.
When the team examined how the molecule behaved in the S1 excited state, they saw unexpected oscillations in the absorption signals during its picosecond-long lifetime. According to Tahara, these signals are unmistakable evidence that the excited complex vibrates coherently in place and waits a short while before distorting.
Because this result contradicts traditional understandings of transition metal processes—atomic movements were theorized to immediately follow excitation to S1-type electronic states—it may spark revolutionary changes in how chemists conceive and control photo-initiated reactions. “This is a fundamental and deep issue,” says Tahara.
By expanding this technique to other poorly understood metal complexes, the team hopes to produce ‘textbook-type’ results that can guide future development of these remarkable materials.
More information: Iwamura, M., et al. Coherent nuclear dynamics in ultrafast photoinduced structural change of bis(diimine)copper(I) complex. Journal of the American Chemical Society 133, 7728–7736 (2011). www.riken.jp/engn/r-world/rese … o/spectro/index.html
Provided by RIKEN