Learning from plants: visible light energy harvesting
How do they do it? Plants make use of only the energy of sunlight for their requirements. Many researchers are trying to mimic the process to harness the vast energy of the sun. In the article published recently in Angew. Chem. Int. Ed.,[1] Jianzhang Zhao et al. of the Dalian University of Technology (China) showed that long-lived triplet excited states are tremendously important for applications in light harvesting. Now they report in the European Journal of Inorganic Chemistry significantly long room-temperature triplet excited state lifetimes for cyclometallated, coumarin-containing IrIII complexes with strong absorption in the visible range.
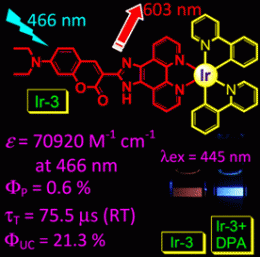
Coumarin, which is known for its intense absorption, is a suitable "antenna" to enhance the absorption of complexes to be used as sensitizers. Light-harvesting RuII–coumarin dyads have been designed previously; however, their absorption is in the UV region. The excitation wavelengths of typical cyclometalated IrIII complexes are in the UV or blue region, and their absorption is weak in the visible range. The addition of a coumarin group to the diimine ligand in a cyclometallated iridium(III) complex increased its absorption in the visible region tremendously. Zhao et al. report a light-harvesting cyclometalated IrIII molecular array with intense absorption in the visible region and a triplet excited state with a lifetime over 25 times longer than those of analogous ruthenium compounds.
Although the dyads are weakly phosphorescent (ΦP = 0.6%), the authors prove that the triplet excited states of the IrIII complexes are efficiently populated upon photoexcitation, by using the complexes as triplet sensitizers for triplet–triplet annihilation upconversion. Upconversion quantum yields (ΦUC) of up to 23.4% were observed. With these results, the authors also question the classical understanding of triplet–triplet annihilation upconversion, which stipulates that quenching of the phosphorescence of the sensitizer should accompany upconverted fluorescence. Upconversion from a weakly phosphorescent excited state opens the way to a completely new approach to the design of light-harvesting complexes.
[1] S. Ji, W. Wu, W. Wu, H. Guo, J. Zhao, Angew. Chem. Int. Ed. 2011, 50, 1626–1629
More information: Jianzhang Zhao et al, Visible-Light Harvesting with Cyclometalated Iridium(III) Complexes Having Long-Lived 3IL Excited States and Their Application in Triplet–Triplet-Annihilation Based Upconversion, European Journal of Inorganic Chemistry, dx.doi.org/10.1002/ejic.201100501
Provided by Wiley