Taming the molecule's Dr. Jekyll and Mr. Hyde
Many organic molecules are non-superimposable with their mirror image. The two forms of such a molecule are called enantiomers and can have different properties in biological systems. The problem is to control which enantiomer you want to produce – a problem that has proved to be important in the pharmaceutical industry. Researchers at the University of Gothenburg have now come up with a new method to control the process.
"Organic chemists think that it's impossible to create only one of the enantiomers without introducing some kind of optical activity into the reaction, but I've succeeded," says Theonitsa Kokoli at the University of Gothenburg's Department of Chemistry. "My method will allow the industry to produce the version they want without the use of a catalyst."
The phenomenon of non-superimposable mirror-image molecular structures is known as chirality. The two enantiomers can be compared to a pair of hands; they are non-superimposable mirror images of each other. A consequence of the different properties in biological systems is that a molecule can behave either as Dr Jekyll or Mr Hyde. The different characteristics in the enantiomers can be harmless, like in the limonene molecule. One enantiomer smells like orange and the other like lemon.
Thalidomide is a good example of how different forms of the same molecule can have disastrous consequences. One of the enantiomers was calming and eased nausea in pregnant women, while the other caused serious damage to the foetus. The thalidomide catastrophe is one of the reasons that a lot of research is devoted to chirality, as it is absolutely vital to be able to control which form of the molecule that is produced. Research on chirality has resulted in several Nobel Prizes over the years.
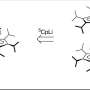
In biomolecules like DNA and proteins only one of the enantiomers exists in nature. In contrast to biomolecules, the same does not apply when chiral compounds are created synthetically in the lab. Generally an equal amount of both enantiomers is produced. One way of creating an excess of one enantiomer is to use a chiral catalyst, but this only transfers the properties that are already present in the catalyst.
"I've been working with absolute asymmetric synthesis instead, where optical activity is created," says Kokoli. "This is considered impossible by many organic chemists. I've used crystals in my reactions, where the two forms have crystallised as separate crystals, which in itself is fairly unusual. The product that was formed after the reactions comprised just one enantiomer."
While the results of Kokoli's research are particularly significant for the pharmaceuticals industry, they can also be used in the production of flavourings and aromas.
Provided by University of Gothenburg