Sugar synthesis hits the sweet spot
A new strategy for synthesizing the kind of complex molecules that certain bacteria use to build their protective cell walls has been developed by Akihiro Ishiwata and Yukishige Ito from the RIKEN Advanced Science Institute in Wako, Japan. The strategy applies to Mycobacterium tuberculosis, the causative agent of tuberculosis (TB), so it could lead to much-needed new medicines to combat the spread of multi-drug-resistant strains of the pathogen.
Disrupting the formation of the cell wall of M. tuberculosis is already a proven strategy for treating TB, with several of the current front-line drugs working in this way. However, the cell wall skeleton is a complex, highly branched structure, and its biosynthesis is not yet fully understood.
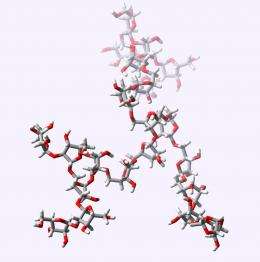
According to Ito, the compound he and Ishiwata made—a sugar-based structure known as the arabinan motif (Araf22) (Fig.1)—should be a useful biological probe, helping to unravel cell wall biosynthesis. Perhaps more importantly, however, the success of their strategy suggests that larger, more complex cell wall components could be made in the same way.
Sugar-based compounds are notoriously difficult to make. Sugars are bristling with reactive alcohol groups, so molecules made from more than 20 sugar units pose a significant synthetic challenge. Nevertheless, Ishiwata and Ito succeeded in clipping together the branching chain of 22 sugar units needed to make Araf22.
Their strategy involved synthesizing small sub-structures of the mycobacterial cell wall skeleton and building from there. To make the compound, they conceptually broke down Araf22’s structure into several simpler fragments, chemically synthesized those fragments, and then clipped them together to make Araf22. This aspect of the strategy has been applied before, but Ishiwata and Ito built the fragments such that they clipped together at linear rather than branching points in their structure.
The researchers’ strategy makes the individual fragments more difficult to build, but it makes the coupling process much more efficient. Crucially, that means the strategy should work just as well as a way to make even larger and more complex components of the cell wall.
“One of the main points of this work is for us to show the way to construct the more complex compounds,” says Ishiwata. “We are now planning to synthesize more complex but structurally reliable glycans of cell wall skeletons for biological studies.” However, such compounds could even prove to be useful drugs in themselves, if they are able to disrupt the cellular machinery responsible for mycobacterial cell wall biosynthesis.
More information: Ishiwata A. & Ito Y. Synthesis of docosasaccharide arabinan motif of mycobacterial cell wall. Journal of the American Chemical Society 133, 2275–2291 (2011)
Provided by RIKEN