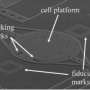
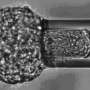
Manipulating cells with a micro-suction cup

Researchers at the Institute for Biomedical Engineering have devised a novel method to pick up and relocate individual cells with a microtip without damaging them. In the future this could be used to verify how robust synthetically grown tissue structures e.g. on implant surfaces are.
Tomaso Zambelli’s research group from the Institute for Biomedical Engineering at ETH Zurich already made a name for itself with a publication a year ago: the team managed to inject substances into individual cells using a microneedle with an aperture of 200 nanometres (about 500 times smaller than the diameter of a strand of human hair).
In collaboration with the ETH Institute of Microbiology two doctoral students, Pablo Dörig and Philipp Stiefel, have now developed a new application for the “fluidic force microscope” (FluidFM) presented in the journal Applied Physics Letters. Based on the technology of an atomic force microscope, they describe a method that can be used to pick up individual viable cells with a hollow needle via a vacuum. The needle has an opening at its apex measuring between 0.5 and six micrometers in diameter. During the whole procedure a laser beam is used to constantly control the force exerted on the cells by the microneedle.
Fishing individual pathogens out of test samples
Using neurons, yeast cells and E.coli bacteria that were only a few micrometers in size, the team proved that they could pick up and displace individual living cells with the system without harming them. With conventional methods, such as tiny glass pipettes for instance, living cells and microbes often become damaged during the treatment. Using the new technique, however, individual bacteria could be removed from a sample with the utmost precision and subsequently be analysed, which has practically been impossible until now. However, the micro suction cup of the FluidFM is also ideal for building up new cell structures, out of individual nerve cells, for example.
Moreover, the researchers believe they have also found another important application for their invention: cellular adhesion, i.e. the force with which a single cell sticks to the surrounding tissue or another substrate. Using the micro suction cup such measurements can be carried out with ease and at an unprecedented rate. “It’s especially consequential to know how strongly the grown cell structures are interconnected in the quality control for synthetic tissues used in implants. Our suction cup enables us to conduct such measurements swiftly and in a standardised fashion”, explains Michael Gabi, who was involved in the project. University Hospital Zurich has already expressed an interest in such a system to control artificial heart valves. The suction cup has now been patented and is to be marketed by the ETH-Zurich start-up company Cytosurge.
More information: Dörig P., Stiefel P. et al.: Force-controlled spatial manipulation of viable mammalian cells and micro-organisms by means of FluidFM technology. Applied Physics Letters 2010; 97. DOI:10.1063/1.3462979
Source: ETH Zurich