This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Nuclear fuel experiment demonstrates how liquid plutonium oxide behaves at the hottest temperatures

The 2011 accident at the Fukushima-Daiichi plant in Japan inspired extensive research and analysis that elevated nuclear energy into a standard bearer for safety. It also inspired a number of studies at the U.S. Department of Energy's (DOE) Argonne National Laboratory. Scientists want to look more closely at nuclear fuel materials to better understand how they will behave at extremely high temperatures.
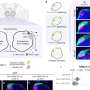
In 2014, using the bright X-rays of beamline 6-ID-D at Argonne's Advanced Photon Source (APS), a DOE Office of Science user facility, a multidisciplinary team measured and published the structure of molten uranium dioxide (UO2). This material is a major component of the fuel used in nuclear reactors around the world. In addition to providing answers, the experiment raised questions about how liquid plutonium oxide (PuO2) and other mixed oxide fuels being considered for use in next-generation reactors would behave at similarly high temperatures.
Experimental study of PuO2 raises many more safety concerns. However, the team at Argonne believed there was a fundamental need for data for actinide oxides. They took on the difficult task of designing an experiment that could overcome the complex challenges that accompany the study of PuO2.
The results of their work will help scientists and engineers model, design and construct clean nuclear energy systems that continue an impressive safety legacy.
The team of Argonne chemical engineers and physicists, in collaboration with Stephen Wilke and Rick Weber of Materials Development, Inc., and others, published the results of their experiment, "Plutonium oxide melt structure and covalency," in the April 2024 issue of Nature Materials.
"Argonne is probably the only place in the world capable of performing this very difficult type of experiment," said Argonne Senior Physicist Chris Benmore. "We demonstrated the proof of principle in 2014 with UO2, and it took us until now to expand the capability to PuO2. The experiments involve complex instrumentation operating at extreme conditions."
Benmore helped design the experimental X-ray chamber, perform the X-ray measurements, and analyze and model the X-ray data. Mark Williamson, division director of Argonne's Chemical and Fuel Cycle Technologies (CFCT) division, assisted in the chamber design and safety analysis for the APS experiments, and guided a CFCT team who synthesized the samples for the experiments. Materials Development, Inc. developed the instrument for the measurements and made necessary safety adjustments that made the instrument more suitable for the PuO2 experiment.
Floated like a butterfly, then stung like a bee
Samples of PuO2 measuring about 2 mm in diameter were levitated on a gas stream and then heated by a carbon dioxide laser beam until they melted. This allowed the team to measure the samples' structure at temperatures as high as 3,000 K without risking sample contamination from container interactions. Samples initially appeared matte gray. After heating, they were shiny black. Heating the sample at different temperatures on different streams of gas revealed changes in the volatility of the melt and structure in various atmospheres.
"We solved the structure of liquid plutonium oxide and found that some covalent bonding was indeed present," Benmore said. "We also discovered that the liquid structure was similar to cerium oxide, which can be used as a non-radioactive substitute."
Lead author Stephen Wilke, of Materials Development Inc., added, "People have used levitators to melt materials at extremely high temperatures, but to move this technique into studying nuclear fuel materials where there are other concerns required a much higher level of sophistication and safety review. I think it paid off in a large way."
Another exciting outcome of the experiment involved using the X-ray data to develop machine learning on a supercomputer at Argonne's Laboratory Computing Resource Center. The team was able to model what all the electrons in the system are doing with quantum mechanical accuracy. This work can shed further light on the nature of bonding mechanisms and help determine the safety parameters of using mixed oxide fuels in future reactors.
"The data from the combined set of experiments not only provides information of technological importance, it also provides insights into the fundamental behavior of actinide oxides at extreme temperatures," Williamson said. "This has been a fantastic collaboration of experts, and it's an excellent example of how we work together to continually improve nuclear energy systems."
More information: Stephen K. Wilke et al, Plutonium oxide melt structure and covalency, Nature Materials (2024). DOI: 10.1038/s41563-024-01883-3
Journal information: Nature Materials
Provided by Argonne National Laboratory