This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Discovery unveils new path to ethanol production from CO₂
In a study published in Energy & Environmental Science, researchers from the Interface Science Department at the Fritz Haber Institute have introduced a novel method for converting the greenhouse gas carbon dioxide (CO2) into ethanol, a sustainable fuel. This significant advancement could pave the way for more environmentally friendly and economically viable alternatives to fossil fuels.
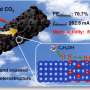
The article, entitled "Time-Resolved Operando Insights into the Tunable Selectivity of Cu-Zn Nanocubes during Pulsed CO2 Electroreduction," reveals how the team successfully used a combination of copper and zinc oxide to favor the catalytic reduction of CO2 into ethanol.
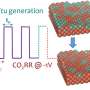
Traditionally, this process has relied solely on copper-based catalysts operated under stationary reaction conditions, which do not ensure the best selectivity for ethanol. Pulsed CO2RR is known to change this, but while it is a promising approach, the catalyst can suffer from stability issues due to the more demanding reaction conditions, which are detrimental to its performance.
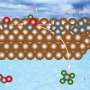
This new research highlights the benefits of using pulsed electrochemical CO2 reduction (CO2RR) techniques. Moreover, the team discovered that by adding a zinc oxide shell to the copper oxide nanocubes, it is possible to increase the production of ethanol while minimizing unwanted by-products such as hydrogen. It was especially possible to achieve similar—if not superior—results in ethanol production with respect to pure Cu catalysts, but with significantly less demanding reaction conditions.
In the past, the oxidation process of the catalyst involved in pulsed CO2 reduction was found to lead to the loss of Cu atoms via oxidative dissolution in the liquid medium (electrolyte), reducing its effectiveness over time. On the contrary, the present study unveiled that a more durable electrocatalyst can be created by design through the deposition of a zinc oxide-coating on the copper nanocubes. Using the new catalysts, it is then the zinc component that primarily oxidizes, sparing the copper and thereby maintaining the catalyst's integrity and efficiency.
This innovative approach therefore enhances the lifetime of the catalysts themselves under the dynamic reaction conditions optimized for the generation of alcohol products. The detailed information on the structure and composition of the catalytic material required for its optimization was obtained via operando Raman spectroscopy, a method with excellent sensitivity for the detection of adsorbed reaction intermediates.
This discovery not only supports the hypothesis that the metal oxidation state plays a crucial role in the reaction and that the active reaction species are created during the catalytic process, but also demonstrates a potential way to enhance the selectivity and efficiency of CO2 reduction to ethanol. It represents a significant step forward in the quest for sustainable energy solutions while offering a promising route for a green and cost-effective production of ethanol and other fuels from CO2.
More information: Antonia Herzog et al, Time-resolved operando insights into the tunable selectivity of Cu–Zn nanocubes during pulsed CO2 electroreduction, Energy & Environmental Science (2024). DOI: 10.1039/D4EE02308K
Journal information: Energy & Environmental Science
Provided by Max Planck Society