This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers decipher new molecular mechanisms related to biological tissue regeneration
A new study published in The EMBO Journal opens new perspectives to better understand how the molecular mechanisms involved in regenerative medicine work.
The study focuses on tumor necrosis factor-α (TNF-α) and its receptors TNFR, molecules of key interest in biomedicine due to their involvement in multiple diseases such as obesity related to type 2 diabetes mellitus, inflammatory bowel disease and several types of cancer.
The study is led by Professor Florenci Serras, from the Faculty of Biology and the Institute of Biomedicine of the University of Barcelona (IBUB). The work also involves experts from the UB's Biodiversity Research Institute (IRBIO), the Center for Genomic Regulation (CRG) and the August Pi i Sunyer Biomedical Research Institute (IDIBAPS).
The work was also highlighted in the News & Views section of the journal in an article by Ditte S. Andersen and Julien Colombani.
The findings indicate that tumor necrosis factor-α (TNF-α)—a cellular activity modulating protein—has two TNFR receptors that can display completely opposite functions in response to biological tissue injury: Specifically, one receptor enhances cell survival and regeneration, while the other can promote cell death.
The study, carried out using the Drosophila melanogaster study model, could contribute to the design of TNFR receptor agonist and antagonist molecules that stimulate the regeneration of epithelial tissues in patients with severe burns, or affected by inflammatory bowel diseases and some cancers.
Drosophila: A model for studying human diseases
Communication between cells is a decisive process in the development and physiology of organisms. One of the pathways of cell communication is the secretion of molecules—e.g., tumor necrosis factor (TNF-α)—that have specific functions in biological cells, tissues and organs.
"In particular, the secreted tumor necrosis factor can recognize and bind to its receptor TNFR, which is located on the membrane of neighboring cells. As a result of the binding, the TNFR receptor is activated and regulates processes as diverse as cell proliferation, cell death and adaptive immunity," explains Serras, a member of the UB's Department of Genetics, Microbiology and Statistics.
In the mammalian genome, there are 19 TNF molecules and 29 TNFR receptors, which reveals the great complexity of their study in the case of the human species. However, some organisms such as the D. melanogaster fly have only one tumor necrosis factor (called Eiger, Egr) and only two TNFRs, which are the Grindelwald (Grnd) and Wengen (Wgn) receptors.
"Thanks to this simplicity, and adding the multiple genetic tools of Drosophila, we have been able to use this model organism to study the regulation and function of TNF-α/TNFR," says the researcher.
Receptors with opposing functions
Although TNF-α and TNFR receptors are linked to acute and chronic diseases, "it is still not well understood how these components regulate such opposing cellular processes as cell death or cell survival, and even cell proliferation," Serras stresses.
This study, which will be included in the doctoral thesis to be defended by Ph.D. student José Esteban-Collado, provides evidence that supports the different and opposing functions of TNFR Grnd and Wgn. "On the one hand, the Grnd receptor promotes cell death (apoptosis) to eliminate damaged cells through a TRAF2-dTAK1-JNK signaling pathway in a TNF-α Egr-dependent manner," says Serras.
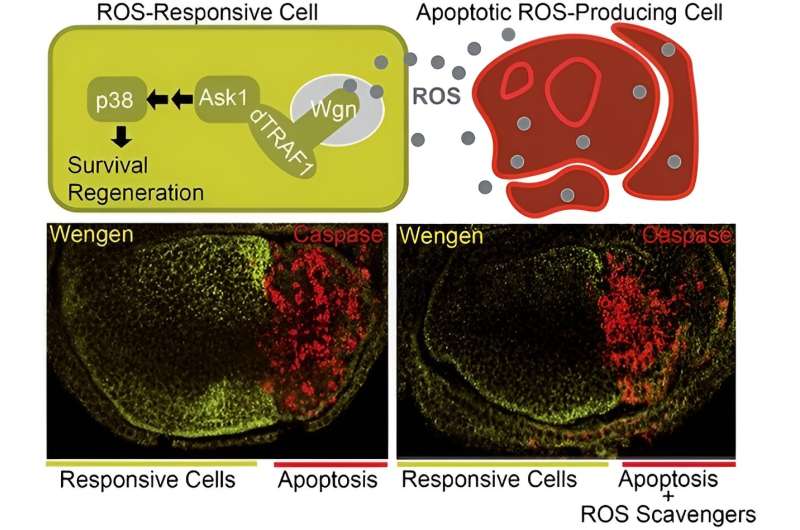
"In contrast, the Wgn receptor promotes cell survival and regeneration to keep tissues healthy and in good condition, via the TRAF1-Ask1-p38 signaling pathway and without the need for TNF-α Egr," he adds.
"That is, the first receptor needs the ligand to bind to the receptor, while the second can be activated without interacting with the ligand. Therefore, each TNFR promotes its signaling to achieve different functions," explains Serras. "Thus, the communication mechanisms of TNFRs must generate a balance between the activities of the different TNFRs, the molecular signals they set in motion and their dependence—or not—on the ligand (TNF-α)."
Damaged cells give off molecular signals in healthy cells
When a cell is dying or damaged, it communicates with healthy cells to replace the non-functional cell with a new one and initiate regeneration of the affected tissue. The research describes how dying cells release reactive oxygen species (ROS), which functional cells in their environment pick up to drive the regeneration process of the affected tissue.
"In a pathological situation or tissue damage, both receptors show different responses. First, the affected tissue produces TNF-α Egr, which binds to Grnd on the membrane. This is internalized and promotes suicide by cell death (apoptosis). At the same time, these cells produce ROS, which spread and reach healthy cells as an alarm signal indicating tissue deterioration," explains Serras.
"The ROS signal activates Wgn in healthy cells directly, without the need for Egr, and consequently triggers the signaling pathway that promotes tissue survival, protection and regeneration," notes Serras.
The results of the new study support the model in which ROS from damaged tissue can activate Wgn-dependent signaling in healthy surrounding cells to promote their regeneration.
Using an elegant binary system that allows manipulation of a gene in tissue-specific domains, the authors have also determined an essential role for TNFR Wgn—but not Grnd—in the activation of p38 kinase. "In healthy cells, this p38 will be responsible for setting in motion the entire genetic machinery for tissue repair," concludes Serras.
More information: José Esteban-Collado et al, Reactive oxygen species activate the Drosophila TNF receptor Wengen for damage-induced regeneration, The EMBO Journal (2024). DOI: 10.1038/s44318-024-00155-9
Ditte S Andersen et al, Wengen's hidden powers: ROS triggers a TNFR-dependent tissue regenerative pathway in Drosophila, The EMBO Journal (2024). DOI: 10.1038/s44318-024-00170-w
Journal information: EMBO Journal
Provided by University of Barcelona