Scientists find a way to postpone cell death
A team of scientists from MSU and the Institute of Theoretical and Experimental Biophysics of Russian Academy of Sciences (located in Pushchino) have studied the mechanisms of interaction between the Fas-ligand protein that causes cell death and a respective membrane receptor. To initiate cell death, the ligand needs to contact with a specific protein component of the cell—caveolin. If the caveolin-binding domain is removed from the molecule of Fas-ligand, cell death may be prevented. The results of the study were published in Cell Death & Disease.
Fas-ligand belongs to a group of tumor necrosis factors. Their main task is to induce cell death, and the process starts after interaction with "death receptors" located on the surface of the plasma membrane. The initial contact triggers a cascade of reactions ending with apoptosis, one of the modes of cell death.
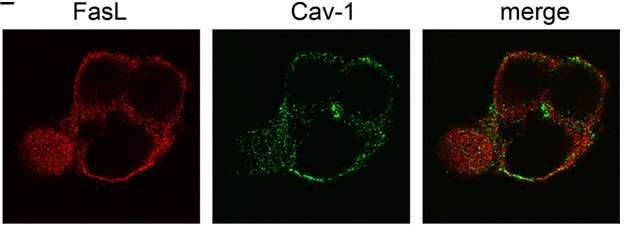
"We studied the mechanisms of the reaction between Fas-ligand and its receptor, and found that for the stimulation of the receptor, the membrane protein caveolin is required. It is attached to Fas-ligand, and if we remove the caveolin-binding domain, the ligand will become considerably less toxic for cells. In our experiments, we used traditional cell and molecular biology methods," explains researcher Vladimir Gogvadze.
In a cell, Fas-ligand is soluble, or is a part of the membrane, either crossing or penetrating through it. In the latter case, a Fas-ligand has extracellular, intracellular, and membrane components. The extracellular component is responsible for receptor recognition, the functions of the membrane component are poorly understood, and the intracellular component is involved in transport and signaling processes, as well as in trafficking the ligand into the membrane rafts formed by molecules of cholesterol and sphingolipids assembled around cavaeolin. To trigger apoptosis, the activated Fas-ligand should be a part of such a raft, which indicates its possible interactions with components of the membrane environment.
Indeed, the transcription of the amino acid sequence of the Fas ligand made it possible to identify the presence of special domains that selectively bind to caveolin. The experiments performed on mutant cells that did not contain such docking fragments confirmed the assumption: Unable to interact with caveolin, the Fas protein lost its killer activity, and its toxicity for cells decreased. It is already known that caveolin is able to suppress tumor development. In the light of the data obtained by the researchers, it can be assumed that a role in this process belongs to the link between caveolin and the Fas ligand—this mechanism causes potential cancer cells to undergo apoptosis.
"The main value of our work is to disclose the mechanisms of cell death stimulation. In the future it will help to develop new strategies for the treatment of cancer," concludes Vladimir Gogvadze.
More information: Xenia A. Glukhova et al, Impairment of Fas-ligand–caveolin-1 interaction inhibits Fas-ligand translocation to rafts and Fas-ligand-induced cell death, Cell Death & Disease (2018). DOI: 10.1038/s41419-017-0109-1
Provided by Lomonosov Moscow State University