This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Researchers present one-pot photothermal upcycling of polylactic acid to hydrogen and pyruvic acid
Plastic waste, a ubiquitous pollutant, poses a significant threat to both humans and the environment. However, it also represents a vast carbon resource. Recycling or upcycling plastic waste is not just a question of environmental sustainability but also a crucial step toward a circular economy.
This topic has sparked considerable interest in the field of catalysis, as the design and construction of catalysts and catalytic processes for upgrading plastic waste could have a profound impact on our planet's health and future.
Photothermal catalysis is a complex synergy that combines photochemical and thermochemical reaction pathways. It involves additional heating to promote the excitation and separation of carriers and accelerate the diffusion of molecules. This intricate process can significantly enhance the performance of the original photocatalytic reaction, but it also presents a host of challenges that researchers in the field of catalysis are eager to tackle.
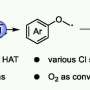
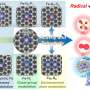
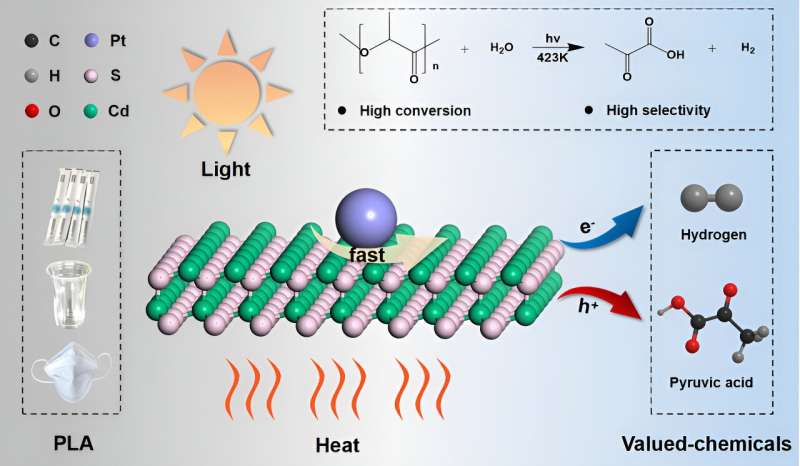
Recently, a research team led by Prof. Fan Zhang from Sichuan University, China, provided a highly efficient Pt/CdS catalyst, realizing the upcycling of polylactic acid to green hydrogen and value-added pyruvic acid through photothermal catalysis. The results were published in Chinese Journal of Catalysis.
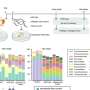
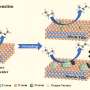
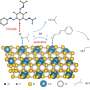
Due to the excellent optical absorption and utilization capability, Pt/CdS possesses superior photocatalytic activity in the degradation of PLA. The effects of external heat on the excitation and utilization of energetic hot carriers over Pt/CdS further improve the activity for upcycling PLA to PyA and H2 in photothermal catalysis. Strong light absorption capability and the suitable valence band edges make Pt/CdS more selective for the production of PyA.
In situ ESR probes the carbon-centered radicals that were preferred intermediates in LA conversion. It proves that the oxidation of LA molecules is initiated by the cleavage of the α-C(sp3)-H bond to form a radical intermediate, which is further oxidized by the holes to produce PyA with hydrogen produced through the reduction of protons.
DFT calculation shows that the high PyA selectivity for LA oxidation on the CdS surface is due to the different affinity of the active sites for C- and O-adsorbed species, resulting in enhanced dehydrogenation and hindered C-C cleavage.
More information: Yuan Xiang et al, One-pot photothermal upcycling of polylactic acid to hydrogen and pyruvic acid, Chinese Journal of Catalysis (2024). DOI: 10.1016/S1872-2067(23)64638-8
Provided by Chinese Academy of Sciences