This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Fixing excess carbon dioxide: Biocatalyst-driven carboxylation under mild conditions
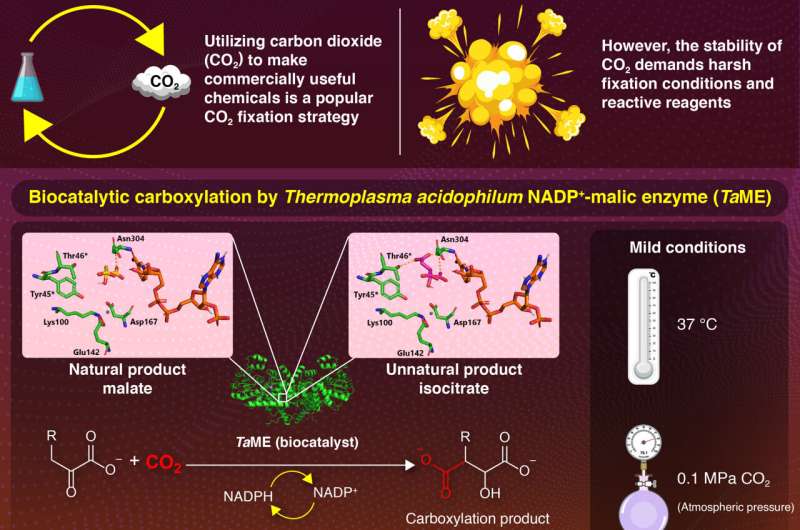
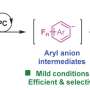
Carbon capture and utilization technologies for the conversion of carbon dioxide into carboxylic acids have garnered attention recently, with researchers from Tokyo Tech recently demonstrating a biocatalyzed carboxylation reaction of not only natural substrate, pyruvate, but also an unnatural one, 2-ketoglutarate, using Thermoplasma acidophilum NADP+- malic enzyme under mild reaction conditions. The proposed strategy can be tailored for the selective synthesis through carbon dioxide fixation reactions.
Removing the excess carbon dioxide (CO2) from the environment is not the end goal of the decarbonization process necessary to reduce the effects of global warming caused by the greenhouse gas. Rather, novel carbon capture and utilization (CCU) technologies are gaining popularity in the current decade as an effective method for removing CO2 from the environment and transforming it into something valuable, for instance, commercially used chemicals such as carboxylic acids.
However, the stability of CO2 makes it unreactive and therefore a difficult starting material for carboxylic acid production. Thus, the resulting carboxylation procedure requires reactive reagents, high temperature and pressure conditions which significantly impact the process's energy cost and sustainability.
To overcome these issues, researchers Associate Professor Tomoko Matsuda and master student Yuri Oku, both from the Department of Life Science and Technology at Tokyo Institute of Technology (Tokyo Tech), explored the use of biocatalysts for CO2 fixation reactions.
The findings of their study were published online in JACS Au on May 13, 2024. The researchers investigated and performed a carboxylation reaction under mild conditions in the presence of biocatalyst Thermoplasma acidophilum NADP+- malic enzyme (TaME) and gaseous CO2 via coupling enzymatic coenzyme regeneration.
The proposed strategy accomplished the carboxylation reaction of not only a natural substrate pyruvate but also an unnatural substrate 2-ketoglutarate.
"The objective of our study was to develop a TaME-catalyzed carboxylation reaction using only gaseous CO2 as a CO2 source and to widen the substrate specificity of TaME for carboxylation," said Matsuda. For the carboxylation reaction, the researchers chose TaME as the enzyme hoping for robustness and ease of handling, similar to other enzymes from T. acidophilum, which were also reported to have high thermal and CO2-pressure stabilities.
For carboxylation of pyruvate, it was treated with TaME and co-enzyme NADPH under 0.1 MPa pressure of CO2. This, however, led to a relatively lower yield. To solve this issue, the researchers added two new co-factors, namely TaGDH (GDH: glucose dehydrogenase) and D-glucose, which resulted in an 18-fold increase in the yield. They also studied the effects of CO2 pressure, pH, and substrate concentration on the carboxylation reaction.
Furthermore, they successfully carried out reductive carboxylation of unnatural substrate, 2-ketoglutarate, to the corresponding product isocitrate by gaseous CO2, TaME, and TaGDH and D-glucose.
The biocatalyst-driven strategy proposed in this study led to successful carboxylation of natural substrate, pyruvate, and unnatural one, 2-ketoglutarate, under mild temperature (37 °C) and pressure conditions (0.1 MPa CO2), thus, lowering the energy burden and increasing the sustainability of the entire CCU process. The effective use of TaME has opened new avenues for selective synthesis of wider carboxylation products using safer and more environmentally friendly reagents instead of harsh chemicals.
"We believe that our proposed method can be re-engineered to perform a wide range of selective carboxylation reactions using renewable resources, under milder reaction conditions, and with less unwanted by-products and waste, unlocking the possibility of biocatalysis for the utilization of carbon dioxide as a starting material," concludes Matsuda.
More information: Yuri Oku et al, Substrate Promiscuity of Thermoplasma acidophilum Malic Enzyme for CO2 Fixation Reaction, JACS Au (2024). DOI: 10.1021/jacsau.4c00290
Journal information: JACS Au
Provided by Tokyo Institute of Technology