Scientists design and construct minimized synthetic carbon fixation cycle
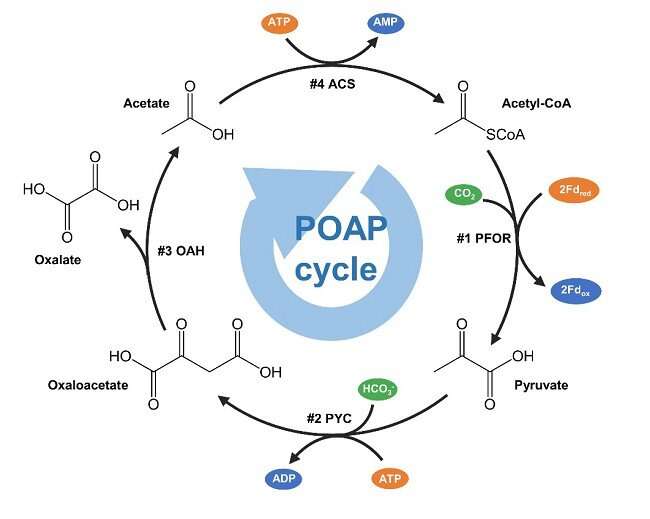
Scientists from the Institute of Microbiology of the Chinese Academy of Sciences (IMCAS) recently reported a minimized synthetic carbon fixation cycle. The cycle only contains four biochemical reactions and is capable of condensing two molecules of carbon dioxide into one molecule of oxalate in each round. The study was published in ACS Catalysis.
The increasing atmospheric carbon dioxide concentration is one of the major contributors to anthropogenic climate change, which has aroused great concern worldwide. In nature, plants and microbes fix carbon dioxide through carbon fixation pathways. Two synthetic carbon fixation pathways, the crotonyl-CoA/ethylmalonyl-CoA/hydroxybutyryl-CoA (CETCH) cycle and the artificial starch anabolic pathway (ASAP) pathway, have also been reported in recent years. However, these pathways usually contain multiple reactions (mostly more than 10 reactions).
Generally, the large number of reactions may reduce the overall carbon fixation efficiency of the pathway. Scientists then asked: is it possible to reduce the number of reactions required for a carbon fixation cycle so as to increase the efficiency? and if possible, what is the minimum number of reactions that can constitute a functional carbon fixation cycle?
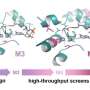
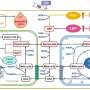
To answer this question, scientists at IMCAS designed a minimized synthetic carbon fixation cycle based on thermodynamic and kinetic calculations of enzymatic reactions. This synthetic cycle contains only four reactions and the four enzymes involved are pyruvate carboxylase (PYC), oxaloacetate acetylhydrolase (OAH), acetate-CoA ligase (ACS) and pyruvate synthase (PFOR), so this cycle was named the pyruvate carboxylase/oxaloacetate acetylhydrolase/acetate-CoA ligase/pyruvate:ferredoxin oxidoreductase (POAP) cycle.
The most challenging reaction in the POAP cycle was the reductive carboxylation of PFOR. Usually, PFOR catalyzes the oxidative decarboxylation of pyruvate. The reductive carboxylation reaction must be driven by a low-potential electron donor. Ferredoxin was selected as the electron donor for this purpose. Among a series of low-potential ferredoxins tested, a ferredoxin from Hydrogenobacter thermophiles was found to efficiently drive the reductive carboxylation of PFOR from Clostridium thermocellum. Subsequently, PYC, OAH and ACS were screened for the construction of POAP cycle.
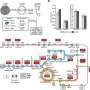
To construct the POAP cycle, four reactions were divided into two cascades, then the two cascades were assembled together, and the carbon fixation efficiency were tested under anaerobic conditions using 13C-labeled NaHCO3 as the substrate. 13C-labeled oxalate produced by the POAP cycle was detected by liquid chromatography–mass spectrometry. The CO2 fixation rate of the POAP cycle was calculated to be 8.0 ± 1.8 nmol CO2 min-1 mg-1 CO2-fixing enzymes and the total turnover number was 5 mol/mol enzymes, surpassing that of the synthetic CETCH cycle.
The POAP cycle functions under relatively high temperature (50 degrees Celsius) and anaerobic conditions, so it may provide a novel model for understanding and investigating CO2 fixation in ancient organisms. The POAP cycle also provides an alternative pathway for biologically converting carbon dioxide into useful chemicals.
More information: Lu Xiao et al, A Minimized Synthetic Carbon Fixation Cycle, ACS Catalysis (2021). DOI: 10.1021/acscatal.1c04151
Journal information: ACS Catalysis
Provided by Chinese Academy of Sciences