This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study identifies new topogenesis pathway for folding and assembly of multi-spanning membrane proteins
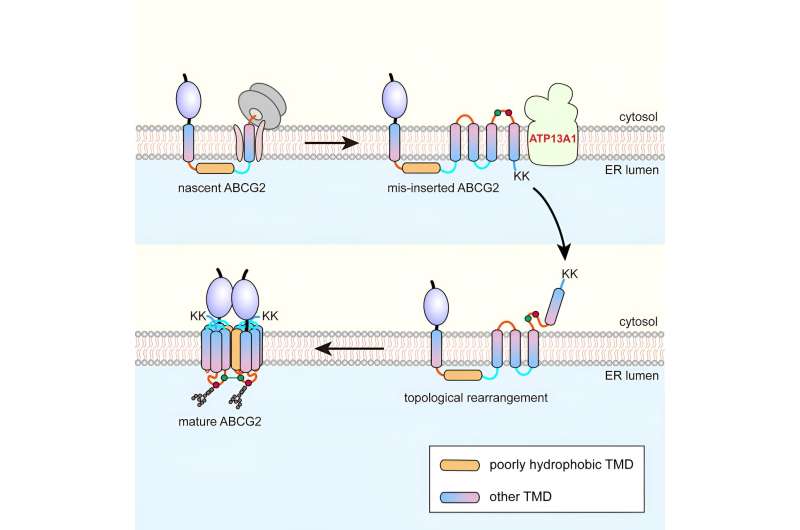
Researchers led by Prof. Zhang Zairong from the Shanghai Institute of Organic Chemistry of the Chinese Academy of Sciences have identified a post-translational topogenesis pathway for the folding and assembly of multi-spanning membrane proteins (MSPs).
Of the approximately 5,000 membrane proteins synthesized at the endoplasmic reticulum (ER) membrane of human cells, more than half are MSPs that play critical roles in cellular and organismal physiology, functioning as ion channels, transporters, receptors, and intramembrane enzymes.
A significant proportion of these functions rely on polar and charged amino acids, leading to the formation of poorly hydrophobic TMDs (pTMDs). However, pTMDs face challenges in being recognized and integrated into the phospholipid bilayer by the Sec61 translocon, which prefers hydrophobic TMDs.
In the human proteome, approximately 30% of membrane proteins and more than 50% of MSPs contain at least one pTMD. How these pTMDs are effectively identified and precisely packaged into mature MSP structures has been a major scientific question.
Using the six-spanning protein adenosine triphosphate-binding cassette transporter G2 (ABCG2) as a model, the researchers found that during co-translational translocation, ABCG2's pTMD2 passes through the central pore of the translocon into the ER lumen, rather than being integrated into the phospholipid bilayer through the translocon's lateral gate.
This results in the insertion of downstream TMDs into the ER membrane with reverse orientations, thereby forming a unique intermediate. Following the translation of the C-terminal positively charged twin lysine residues, a near-global topological rearrangement process occurs.
Affinity purification showed that ATP13A1 can detect the C-terminal positive charge signal of ABCG2. Replacement of lysine residues with negatively charged or neutral amino acids significantly attenuates the interactions between ATP13A1 and ABCG2 mutants.
Furthermore, knockout of ATP13A1 resulted in the apparent accumulation of misfolded ABCG2 conformations, primarily those with misoriented TMD6 within the ER membrane. Thus, ATP13A1 plays a crucial role in the topogenesis of MSPs, where its ATPase activity promotes the dislocation of the misoriented TMD6 from the lipid bilayer into the cytosol.
Subsequently, the cytosolic TMD6 is reintegrated into the ER membrane, thereby driving the post-translational topological rearrangement of other upstream TMDs.
Upon successful rearrangement of TMDs 4-6, the intermediate can oligomerize into a quaternary structure. This process is likely to facilitate the integration of pTMD2 into the final structure from the aqueous ER lumen and into the mature structure, which is tightly wrapped by surrounding TMDs.
In summary, the study, now published in Molecular Cell, explains how a "difficult" pTMD is co-translationally skipped for insertion and post-translationally buried into the final correct structure at the late folding stage, thus avoiding excessive lipid exposure.
Notably, due to the exposure of pTMD2 to the ER lumen during the ABCG2 topogenesis, the N441 glycosylation modification caused by the ABCG2-S441N genetic mutation can significantly block pTMD assembly at the late stage of topogenesis. As ABCG2 is a uric acid transporter, this study explains how this mutation is closely associated with human diseases such as gout and hyperuricemia.
More information: Jia Ji et al, An ATP13A1-assisted topogenesis pathway for folding multi-spanning membrane proteins, Molecular Cell (2024). DOI: 10.1016/j.molcel.2024.04.010
Journal information: Molecular Cell
Provided by Chinese Academy of Sciences