This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Life's insiders: Decoding endosymbiosis with mathematics
Endosymbiosis, the intimate and long-term relationship where one organism lives inside another, is a cornerstone of life as we know it, and a key to the emergence of complex life on Earth. Many of the mysteries surrounding endosymbiosis are difficult to tackle using empirical approaches alone.
In a recent essay published in PLOS Biology, a team of researchers from Umeå University describe how mathematical models can advance endosymbiosis research.
Endosymbionts are everywhere: Within our cells mitochondria generate most of our energy, plants rely on chloroplasts for photosynthesis, and many insects can't reproduce without their endosymbionts. This is, however, just the tip of the iceberg when it comes to endosymbioses.
"Endosymbiotic relationships are incredibly diverse and complex. For instance, new research has revealed that endosymbionts can determine whether embryos can be successfully formed, and even guide embryonic development," says Lucas Santana Souza, postdoctoral fellow at Umeå University and co-author of the article in PLOS Biology.
Despite their ubiquity, endosymbioses can be difficult to study.
"Consider the origin of the mitochondria in our cells. It used to be a separate organism but through an endosymbiosis that happened hundreds of millions of years ago it became a crucial part of all complex life. However, we can't study this ancient and rare event by reproducing it in the lab or going back in time—we need other ways and mathematical models are a great tool," says Eric Libby, Associate Professor at the Department of Mathematics and Mathematical Statistics.
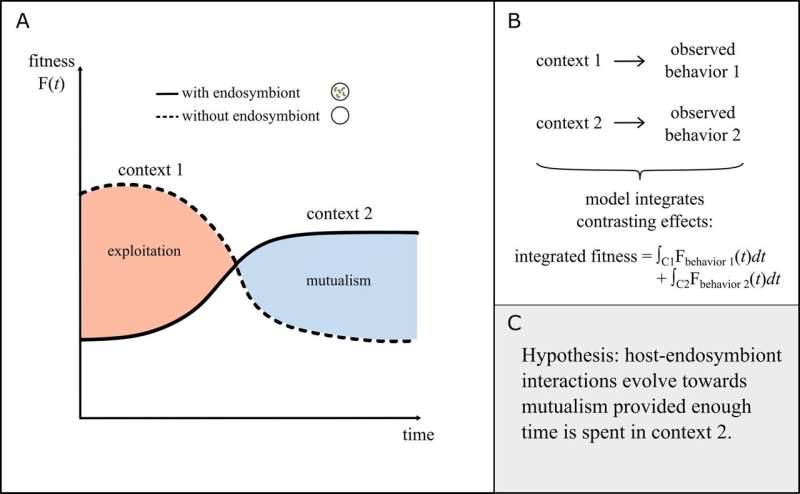
Mathematical models can help us understand how different factors affect the interactions between endosymbionts and their hosts. In the essay, the authors shows how these models can generate ideas and complement real-world research. They also point to important questions for further investigation.
One such example is connected to corals and their endosymbionts, of particular relevance at present as coral bleaching events increase worldwide due to increased heat waves. In coral bleaching, the coral expels its endosymbionts and loses its ability to generate food, which can lead to its death.
Interestingly, corals can switch their endosymbionts to ones that improve their ability to resist heat waves. This is one of the research areas study co-author Adriano Bonforti, postdoctoral fellow at Umeå University, is most interested in.
"The puzzle, is understanding when corals should modify their endosymbiotic community so that one type of endosymbiont becomes dominant over the others, thereby changing the coral's response to stress effectors. Mathematical models can suggest likely reasons for when and how corals should switch. The results of these theoretical approaches can then help guide future experimental research," he says.
The authors also make a case for increased collaboration between endosymbiosis researchers. They draw parallels between endosymbiotic relationships and the interaction between mathematical modelers and experimentalists. Both have different approaches and backgrounds, but the outcome of their collaboration can be enormously fruitful, according to them.
"Think of modelers as beneficial partners, drawing inspiration and posing intriguing questions from the rich empirical discoveries. In this context, modelers contribute by simplifying complex concepts, uncovering fundamental processes, and opening new avenues for exploration. With this essay, we hope to build a stronger bridge between both fields and to indicate fruitful directions for endosymbiotic research," says Lucas Santana Souza.
More information: Lucas Santana Souza et al, Modeling endosymbioses: Insights and hypotheses from theoretical approaches, PLOS Biology (2024). DOI: 10.1371/journal.pbio.3002583
Journal information: PLoS Biology
Provided by Umea University